Microbiology usually is concerned with organisms so small they cannot be seen distinctly with the unaided eye. Because of the nature of this discipline, the microscope is of crucial importance. Thus it is important to understand how the microscope works and the way in which specimens are prepared for examination.
The chapter begins with a detailed treatment
of the standard bright-field microscope and then describes other common types of
light microscopes. Next preparation and staining of specimens for examination
with the light microscope are discussed. This is followed by a description of
transmission and scanning electron microscopes, both of which are used
extensively in current microbiological research. The chapter closes with a
brief introduction to two newer forms of microscopy: scanning probe microscopy and
confocal microscopy.
2.1 Lenses and the Bending of Light
To understand how a light microscope
operates, one must know something about the way in which lenses bend and focus
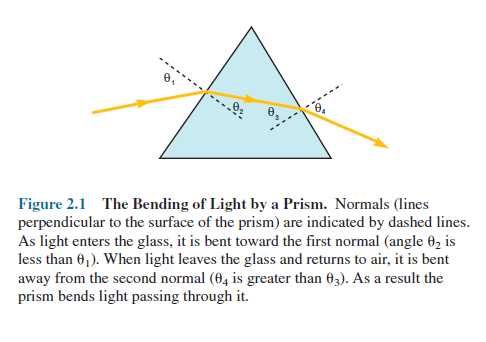
light to form images. When a ray of light passes from one medium to another, refraction occurs—that is, the ray is bent at the interface.
The refractive index is a measure of how greatly a substance slows the
velocity of light, and the direction and magnitude of bending is determined by
the refractive indexes of the two media forming the interface. When light
passes from air into glass, a medium with a greater refractive index, it is
slowed and bent toward the normal, a line perpendicular to the surface (figure 2.1).
As light leaves glass and returns to air, a
medium with a lower refractive index, it accelerates and is bent away from the
normal.
Thus a prism bends light because glass has a
different refractive index from air, and the light strikes its surface at an
angle. Lenses act like a collection of prisms operating as a unit. When the
light source is distant so that parallel rays of light strike the lens, a
convex lens will focus these rays at a specific point, the focal point (F in figure 2.2). The distance between the center of the lens and the
focal point is called the focal length (f in figure 2.2).
Our eyes cannot focus on objects nearer than
about 25 cm or 10 inches (table 2.1). This
limitation may be overcome by using a convex lens as a simple magnifier (or
microscope) and holding it close to an object. A magnifying glass provides a clear
image at much closer range, and the object appears larger. Lens strength is
related to focal length; a lens with a short focal length will magnify an
object more than a weaker lens having a longer focal length.
2.2
The Light Microscope
Microbiologists currently employ a variety of
light microscopes in their work; bright-field, dark-field, phase-contrast, and
fluorescence microscopes are most commonly used. Modern microscopes are all compound
microscopes. That is, the magnified image formed by the objective lens is
further enlarged by one or more additional lenses.
The
Bright-Field Microscope
The ordinary microscope is called a bright-field
microscope because it forms a dark image against a
brighter background. The microscope consists of a sturdy metal body or stand
composed of a base and an arm to which the remaining parts are attached (figure 2.3). A light source, either a mirror or an electric
illuminator, is located in the base. Two focusing knobs, the fine and coarse
adjustment knobs, are located on the arm and can move either the stage or the
nosepiece to focus the image.
The stage is positioned about halfway up the
arm and holds microscope slides by either simple slide clips or a mechanical stage
clip. A mechanical stage allows the operator to move a slide around smoothly
during viewing by use of stage control knobs.
The substage condenser is mounted within or beneath the stage and focuses a cone
of light on the slide. Its position often is fixed in simpler microscopes but
can be adjusted vertically in more advanced models.
The curved upper part of the arm holds the
body assembly, to which a nosepiece and one or more eyepieces or oculars are attached.
More advanced microscopes have eyepieces for both eyes and are called binocular
microscopes. The body assembly itself contains a series of mirrors and prisms
so that the barrel holding the eyepiece may be tilted for ease in viewing (figure 2.4). The nosepiece holds three to five objectives with lenses of differing magnifying power and can be
rotated to position any objective beneath the body assembly. Ideally a microscope
should be parfocal—that is, the image should remain in focus when objectives are
changed.
The path of light through a bright-field microscope
is shown in figure 2.4. The objective lens forms an enlarged real image within
the microscope, and the eyepiece lens further magnifies this primary image.
When one looks into a microscope, the enlarged specimen image, called the
virtual image, appears to lie just beyond the stage about 25 cm away. The total
magnification is calculated by multiplying the objective and eyepiece
magnifications together. For example, if a 45X objective is used with a 10X eyepiece,
the overall magnification of the specimen will be 450X.
Microscope Resolution
The most important part of the microscope is
the objective, which must produce a clear image, not just a magnified one. Thus
resolution is extremely important. Resolution is the ability of a lens to
separate or distinguish between small objects that are close together.
Much of the optical theory underlying
microscope design was developed by the German physicist Ernst Abbe in the
1870s. The minimum distance (d) between two objects that reveals them as
separate entities is given by the Abbe equation, in which lambda (λ) is the
wavelength of light used to illuminate the specimen and n sin Ɵ is the
numerical aperture (NA).
As d becomes smaller, the resolution
increases, and finer detail can be discerned in a specimen. The preceding
equation indicates that a major factor in resolution is the wavelength of light
used. The wavelength must be shorter than the distance between two objects or
they will not be seen clearly. Thus the greatest resolution is obtained with
light of the shortest wavelength, light at the blue end of the visible spectrum
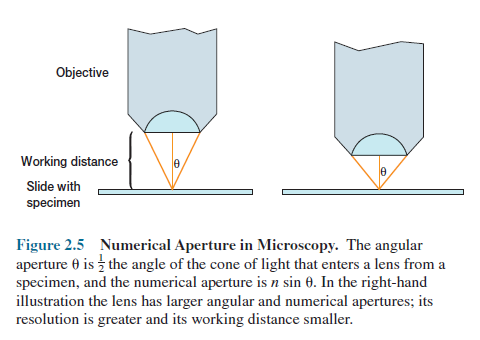
(in the range of 450 to 500 nm). The numerical aperture (n sin Ɵ) is
more difficult to understand. Theta is defined as 1 2 the angle of the cone of
light entering an objective (figure 2.5). Light that strikes the microorganism after
passing through a condenser is cone-shaped.
When this cone has a narrow angle and tapers
to a sharp point, it does not spread out much after leaving the slide and
therefore does not adequately separate images of closely packed objects.
The resolution is low. If the cone of light
has a very wide angle and spreads out rapidly after passing through a specimen,
closely packed objects appear widely separated and are resolved.
The angle of the cone of light that can enter
a lens depends on the refractive index (n) of the medium in which the lens
works, as well as upon the objective itself. The refractive index for air is
1.00. Since sin Ɵ cannot be greater than 1 (the maximum Ɵ is 90° and sin 90° is
1.00), no lens working in air can have a numerical aperture greater than 1.00.
The only practical way to raise the numerical aperture above 1.00, and
therefore achieve higher resolution, is to increase the refractive index with
immersion oil, a colorless liquid with the same refractive index as glass (table
2.2). If air is replaced with immersion oil, many light rays that did not enter
the objective due to reflection and refraction at the surfaces of the objective
lens and slide will now do so (figure 2.6). An increase in numerical aperture
and resolution results.
The resolution of a microscope depends upon
the numerical aperture of its condenser as well as that of the objective. This
is evident from the equation describing the resolution of the complete microscope.
Most microscopes have a condenser with a
numerical aperture between 1.2 and 1.4. However, the condenser numerical
aperture will not be much above about 0.9 unless the top of the condenser is
oiled to the bottom of the slide. During routine microscope operation, the
condenser usually is not oiled and this limits the overall resolution, even
with an oil immersion objective.
The limits set on the resolution of a light
microscope can be calculated using the Abbe equation. The maximum theoretical
resolving power of a microscope with an oil immersion objective (numerical
aperture of 1.25) and blue-green light is approximately 0.2 µm.
At best, a bright-field microscope can
distinguish between two dots around 0.2 µm apart (the same size as a very small
bacterium).
Normally a microscope is equipped with three
or four objectives ranging in magnifying power from 4X to 100X (table 2.2).
The working distance of an objective
is the distance between the front surface of the lens and the surface of the
cover glass (if one is used) or the specimen when it is in sharp focus.
Objectives with large numerical apertures and great resolving power have short working
distances.
The largest useful magnification increases
the size of the smallest resolvable object enough to be visible. Our eye can
just detect a speck 0.2 mm in diameter, and consequently the useful limit of
magnification is about 1,000 times the numerical aperture of the objective
lens. Most standard microscopes come with 10X eyepieces and have an upper limit
of about 1,000X with oil immersion.
A 15X eyepiece may be used with good objectives
to achieve a useful magnification of 1,500X. Any further magnification increase
does not enable a person to see more detail. A light microscope can be built to
yield a final magnification of 10,000X, but it would simply be magnifying a
blur. Only the electron microscope provides sufficient resolution to make
higher magnifications useful.
Proper specimen illumination also is
extremely important in determining resolution. A microscope equipped with a
concave mirror between the light source and the specimen illuminates the slide
with a fairly narrow cone of light and has a small numerical aperture.
Resolution can be improved with a substage condenser, a large light-gathering
lens used to project a wide cone of light through the slide and into the
objective lens, thus increasing the numerical aperture.
The Dark-Field Microscope
Living, unstained cells and organisms can be
observed by simply changing the way in which they are illuminated. A hollow cone
of light is focused on the specimen in such a way that unreflected and
unrefracted rays do not enter the objective. Only light that has been reflected
or refracted by the specimen forms an image (figure 2.7). The field
surrounding a specimen appears black, while the object itself is brightly
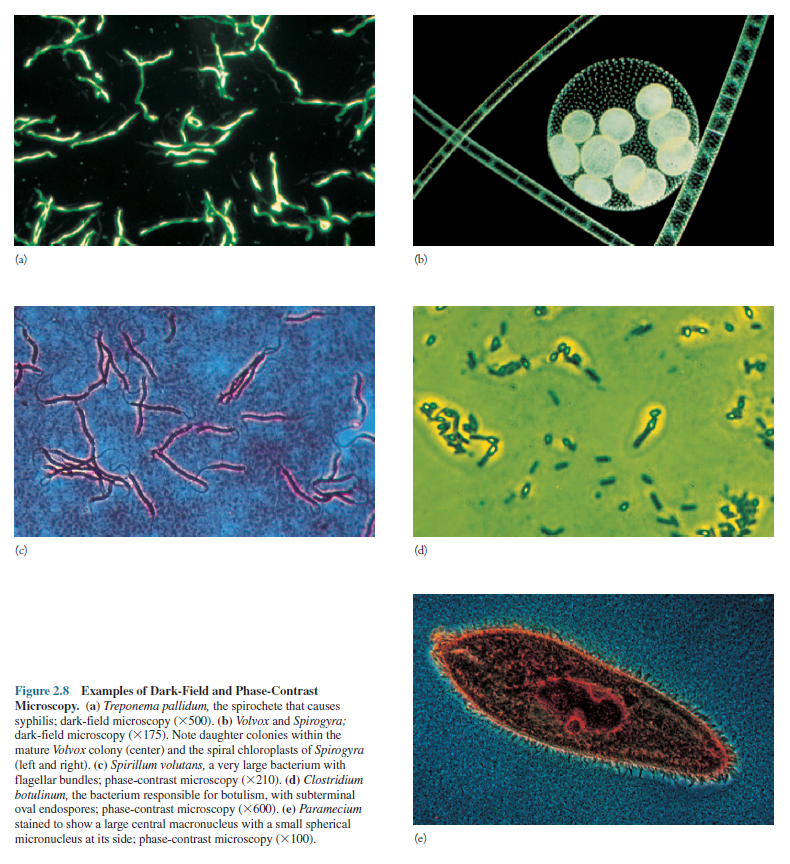
illuminated (figure 2.8a, b); because the background is dark,
this type of microscopy is called dark-field microscopy. Considerable
internal structure is often visible in larger eucaryotic microorganisms (figure
2.8b). The dark-field microscope is used to identify bacteria like the
thin and distinctively shaped Treponema pallidum (figure 2.8a),
the causative agent of syphilis.
The Phase-Contrast Microscope
Unpigmented living cells are not clearly
visible in the brightfield microscope because there is little difference in
contrast between the cells and water. Thus microorganisms often must be fixed
and stained before observation to increase contrast and create variations in
color between cell structures. A phase-contrast microscope converts
slight differences in refractive index and cell density into easily detected
variations in light intensity and is an excellent way to observe living cells (figure
2.8c–e).
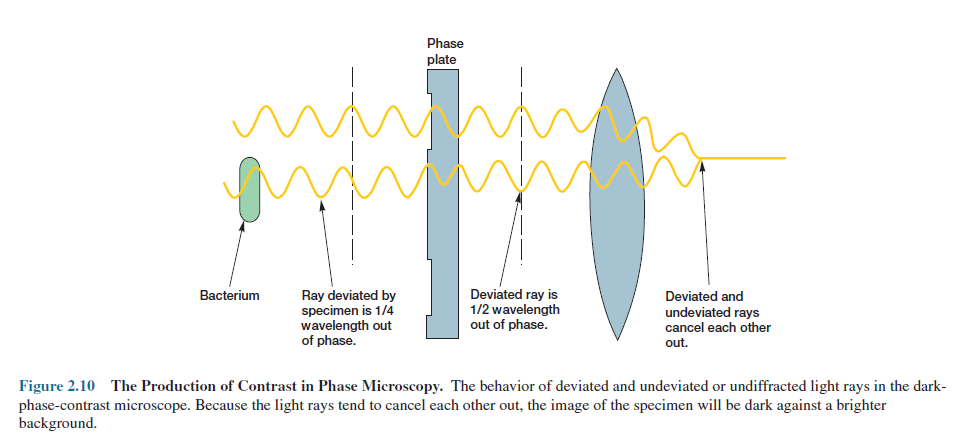
The condenser of a phase-contrast microscope has an annular stop,
an opaque disk with a thin transparent ring, which produces a hollow cone of
light (figure 2.9). As this cone passes through a cell, some light rays
are bent due to variations in density and refractive index within the specimen
and are retarded by about 1/4 wavelength. The deviated light is focused to form
an image of the object. Un-deviated light rays strike a phase ring in the phase
plate, a special optical disk located in the objective, while the deviated rays
miss the ring and pass through the rest of the plate. If the phase ring is
constructed in such a way that the un-deviated light passing through it is
advanced by 1/4 wavelength, the deviated and un-deviated waves will be about 1/2
wavelength out of phase and will cancel each other when they come together to
form an image (figure 2.10). The background, formed by undeviated light,
is bright, while the unstained object appears dark and well-defined.
This type of microscopy is called dark-phase-contrast
microscopy. Color filters often are used to improve the image (figure 2.8c,d).
Phase-contrast microscopy is especially useful for studying microbial
motility, determining the shape of living cells, and detecting bacterial components
such as endospores and inclusion bodies that contain poly-hydroxybutyrate,
polymetaphosphate, sulfur, or other substances.
These are clearly visible (figure 2.8d) because they have
refractive indexes markedly different from that of water. Phase contrast microscopes
also are widely used in studying eukaryotic cells.
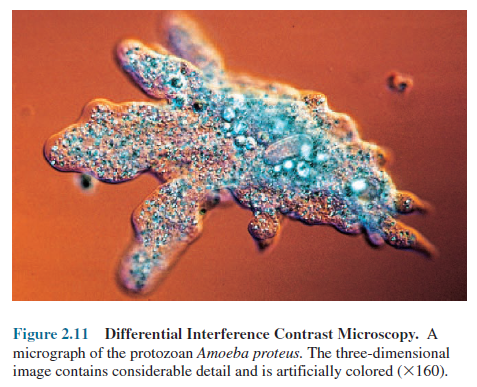
The
Differential Interference Contrast Microscope
The differential interference contrast (DIC)
microscope is similar to the phase-contrast microscope
in that it creates an image by detecting differences in refractive indices and
thickness. Two beams of plane polarized light at right angles to each other are
generated by prisms. In one design, the object beam passes through the
specimen, while the reference beam passes through a clear area of the slide.
After passing through the specimen, the two beams are combined and interfere
with each other to form an image. A live, unstained specimen appears brightly
colored and three-dimensional (figure 2.11). Structures
such as cell walls, endospores, granules, vacuoles, and eucaryotic nuclei are clearly
visible.
The
Fluorescence Microscope
The microscopes thus far considered produce
an image from light that passes through a specimen. An object also can be seen
because it actually emits light, and this is the basis of fluorescence microscopy.
When some molecules absorb radiant energy, they become excited and later
release much of their trapped energy as light. Any light emitted by an excited
molecule will have a longer wavelength (or be of lower energy) than the
radiation originally absorbed. Fluorescent light is emitted
very quickly by the excited molecule as it gives up its trapped energy and
returns to a more stable state.
The fluorescence microscope (figure 2.12) exposes a
specimen to ultraviolet, violet, or blue light and forms an image of the object
with the resulting fluorescent light. A mercury vapor arc lamp or other source
produces an intense beam, and heat transfer is limited by a special infrared
filter. The light passes through an exciter filter that transmits only the
desired wavelength. A darkfield condenser provides a black background against
which the fluorescent objects glow. Usually the specimens have been stained
with dye molecules, called fluorochromes, that
fluoresce brightly upon exposure to light of a specific wavelength, but some
microorganisms are autofluorescing. The microscope forms an image of the
fluorochrome-labeled microorganisms from the light emitted when they fluoresce
(figure 2.13). A barrier filter positioned after the objective lenses removes
any remaining ultraviolet light, which could damage the viewer’s eyes, or blue
and violet light, which would reduce the image’s contrast.
The fluorescence microscope has become an
essential tool in medical microbiology and microbial ecology. Bacterial pathogens
(e.g., Mycobacterium tuberculosis, the cause of tuberculosis) can be
identified after staining them with fluorochromes or specifically labeling them
with fluorescent antibodies using immunofluorescence procedures. In ecological studies
the fluorescence microscope is used to observe microorganisms stained with
fluorochrome-labeled probes or fluorochromes such as acridine orange and DAPI
(diamidino-2- phenylindole, a DNA-specific stain). The stained organisms will fluoresce
orange or green and can be detected even in the midst of other particulate
material. It is even possible to distinguish live bacteria from dead bacteria
by the color they fluoresce after treatment with a special mixture of stains
(figure 2.13d). Thus the microorganisms can be viewed and directly
counted in a relatively undisturbed ecological niche.
2.3
Preparation and Staining of Specimens
Although living microorganisms can be
directly examined with the light microscope, they often must be fixed and
stained to increase visibility, accentuate specific morphological features, and
preserve them for future study.
Fixation of smear
The stained cells seen in a microscope should
resemble living cells as closely as possible. Fixation is the
process by which the internal and external structures of cells and
microorganisms are preserved and fixed in position. It inactivates enzymes that
might disrupt cell morphology and toughens cell structures so that they do not
change during staining and observation. A microorganism usually is killed and
attached firmly to the microscope slide during fixation.
There are two fundamentally different types
of fixation.
(1) Bacteriologists heat-fix bacterial smears by gently flame heating an air-dried film of bacteria. This adequately preserves overall morphology but not structures within cells.
(2) Chemical fixation must be used to protect fine cellular substructure and the morphology of larger, more delicate microorganisms.
Chemical fixatives penetrate cells and
react with cellular components, usually proteins and lipids, to render them
inactive, insoluble, and immobile. Common fixative mixtures contain such
components as ethanol, acetic acid, mercuric chloride, formaldehyde, and
glutaraldehyde.
Dyes
and Simple Staining
The many types of dyes used to stain
microorganisms have two features in common.
(1) They have chromophore groups, groups with conjugated double bonds that give the dye its color.
(2) They can bind with cells by ionic, covalent, or hydrophobic bonding. For example, a positively charged dye binds to negatively charged structures on the cell.
Ionizable dyes may be divided into two
general classes based on the nature of their charged group.
1. Basic dyes—methylene blue, basic fuchsin, crystal violet, safranin, malachite green—have positively charged groups (usually some form of pentavalent nitrogen) and are generally sold as chloride salts. Basic dyes bind to negatively charged molecules like nucleic acids and many proteins. Because the surfaces of bacterial cells also are negatively charged, basic dyes are most often used in bacteriology.
2. Acid dyes—eosin, rose bengal, and acid fuchsin—possess negatively charged groups such as carboxyls (—COOH) and phenolic hydroxyls (—OH). Acid dyes, because of their negative charge, bind to positively charged cell structures.
The pH may alter staining effectiveness since the nature and degree of the charge on cell components change with pH. Thus anionic dyes stain best under acidic conditions when proteins and many other molecules carry a positive charge; basic dyes are most effective at higher pHs.
Although ionic interactions are probably the
most common means of attachment, dyes also bind through covalent bonds or because
of their solubility characteristics. For instance, DNA can be stained by the
Feulgen procedure in which Schiff’s reagent is covalently attached to its
deoxyribose sugars after hydrochloric acid treatment. Sudan III (Sudan Black)
selectively stains lipids because it is lipid soluble but will not dissolve in
aqueous portions of the cell.
Microorganisms often can be stained very
satisfactorily by simple staining, in which a single staining agent is
used. Simple staining’s value lies in its simplicity and ease of use. One covers
the fixed smear with stain for the proper length of time, washes the excess
stain off with water, and blots the slide dry.
Basic dyes like crystal violet, methylene
blue, and carbol fuchsin are frequently used to determine the size, shape, and arrangement
of bacteria.
Differential Staining
Differential staining procedures divide bacteria into separate groups based on staining
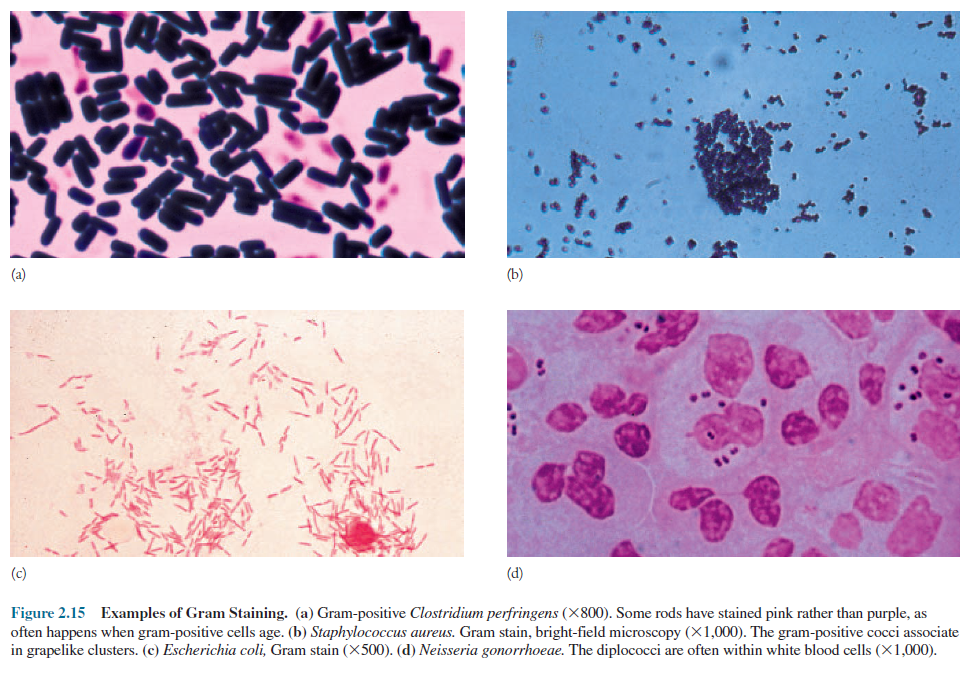
properties. The Gram stain, developed in 1884 by the Danish physician
Christian Gram, is the most widely employed staining method in bacteriology. It
is a differential staining procedure because it divides bacteria into two classes—gram
negative and gram positive. Gram-positive and gram-negative bacteria.
In the first step of the Gram-staining
procedure (figure 2.14), the smear is stained with the basic dye crystal
violet, the primary stain. It is followed by treatment with an iodine solution functioning
as a mordant. That is, the iodine increases the interaction between the
cell and the dye so that the cell is stained more strongly. The smear is next
decolorized by washing with ethanol or acetone. This step generates the
differential aspect of the Gram stain; gram-positive bacteria retain the
crystal violet, whereas gram-negative bacteria lose their crystal violet and
become colorless. Finally, the smear is counterstained with a simple, basic dye
different in color from crystal violet.
Safranin, the most common counter-stain,
colors gram-negative bacteria pink to red and leaves gram-positive bacteria
dark purple (figure 2.15). Cell wall structure and the mechanism of the
Gram stain.
Acid-fast staining is another important differential staining procedure. A
few species, particularly those in the genus Mycobacterium (see
chapter 24) do not bind simple stains readily and must be stained by a
harsher treatment: heating with a mixture of basic fuchsin and phenol (the
Ziehl-Neelsen method).
Once basic fuchsin has penetrated with the
aid of heat and phenol, acid-fast cells are not easily decolorized by an
acid-alcohol wash and hence remain red. This is due to the quite high lipid
content of acid-fast cell walls; in particular, mycolic acid—a group of
branched chain hydroxy lipids—appears responsible for acid-fastness.
Non-acid-fast bacteria are decolorized by
acid-alcohol and thus are stained blue by methylene blue counter-stain. This method
is used to identify Mycobacterium tuberculosis and M. leprae (figure
2.16), the pathogens responsible for tuberculosis and leprosy,
respectively.
Staining Specific Structures of bacteria
Many special staining procedures have been
developed over the years to study specific bacterial structures with the light
microscope. One of the simplest is negative staining, a technique that reveals the presence of the diffuse
capsules surrounding many bacteria. Bacteria are mixed with India ink or
Nigrosin dye and spread out in a thin film on a slide. After air-drying,
bacteria appear as lighter bodies in the midst of a blue-black background because
ink and dye particles cannot penetrate either the bacterial cell or its
capsule. The extent of the light region is determined by the size of the
capsule and of the cell itself. There is little distortion of bacterial shape,
and the cell can be counterstained for even greater visibility (figure 2.17).
Bacteria in the genera Bacillus and Clostridium
form an exceptionally resistant structure capable of surviving for long periods
in an unfavorable environment. This dormant structure is called an endospore
since it develops within the cell.
Endospore morphology and location vary with
species and often are valuable in identification; endospores may be spherical
to elliptical and either smaller or larger than the diameter of the parent bacterium.
They can be observed with the phase-contrast microscope or negative staining.
Endospores are not stained well by most dyes, but once stained, they strongly
resist decolorization. This property is the basis of most spore staining methods
(figure 2.18).
In the Schaeffer-Fulton procedure, endospores
are first stained by heating bacteria with malachite green, which is a very
strong stain that can penetrate endospores. After malachite green treatment,
the rest of the cell is washed free of dye with water and is counterstained with
safranin. This technique yields a green endospore resting in a pink to red
cell. Bacterial endospore structure.
Bacterial flagella are fine, threadlike
organelles of locomotion that are so slender (about 10 to 30 nm in diameter)
they can only be seen directly using the electron microscope. To observe them
with the light microscope, the thickness of flagella is increased by coating
them with mordants like tannic acid and potassium alum, and they are stained
with pararosaniline (Leifson method) or basic fuchsin (Gray method). Flagella
staining procedures provide taxonomically valuable information about the presence
and distribution pattern of flagella (figure 2.19).
2.4 Electron Microscopy
For centuries the light microscope has been
the most important instrument for studying microorganisms. The electron
microscope now has transformed microbiology and added immeasurably to our
knowledge. The nature of the electron microscope and the ways in which
specimens are prepared for observation are reviewed briefly in this section.
The Transmission Electron Microscope
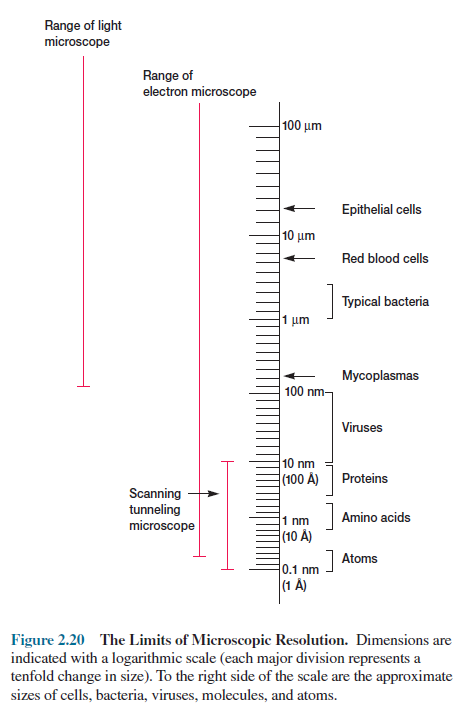
The very best light microscope has a
resolution limit of about 0.2 µm. Because bacteria usually are
around 1 µm in diameter, only their general shape and major
morphological features are visible in the light microscope. The detailed
internal structure of larger microorganisms also cannot be effectively studied
by light microscopy. These limitations arise from the nature of visible light
waves, not from any inadequacy of the light microscope itself.
Recall that the resolution of a light
microscope increases with a decrease in the wavelength of the light it uses for
illumination. Electron beams behave like radiation and can be focused much as
light is in a light microscope. If electrons illuminate the specimen, the
microscope’s resolution is enormously increased because the wavelength of the
radiation is around 0.005 nm, approximately 100,000 times shorter than that of
visible light. The transmission electron microscope has a practical resolution
roughly 1,000 times better than the light microscope; with many electron
microscopes, points closer than 5 Å or 0.5 nm can be distinguished, and the
useful magnification is well over 100,000X (figure 2.20). The value of the electron microscope is evident on
comparison of the photographs in figure 2.21; microbial
morphology can now be studied in great detail.
A modern transmission electron microscope (TEM) is complex and sophisticated (figure 2.22), but the basic principles behind its operation can be
understood readily. A heated tungsten filament in the electron gun generates a
beam of electrons that is then focused on the specimen by the condenser (figure
2.23). Since electrons cannot pass through a glass lens, doughnut-shaped
electromagnets called magnetic lenses are used to focus the beam. The column
containing the lenses and specimen must be under high vacuum to obtain a clear
image because electrons are deflected by collisions with air molecules. The
specimen scatters electrons passing through it, and the beam is focused by
magnetic lenses to form an enlarged, visible image of the specimen on a
fluorescent screen.
A denser region in the specimen scatters more
electrons and therefore appears darker in the image since fewer electrons
strike that area of the screen. In contrast, electron-transparent regions are brighter.
The screen can also be moved aside and the image captured on photographic film
as a permanent record.
Specimen Preparation for Electron Microscopy
Table 2.3 compares some of the important features of light and electron
microscopes. The distinctive features of the TEM place harsh restrictions on
the nature of samples that can be viewed and the means by which those samples
must be prepared. Since electrons are quite easily absorbed and scattered by
solid matter, only extremely thin slices of a microbial specimen can be viewed
in the average TEM. The specimen must be around 20 to 100 nm thick, about 1⁄50 to
1⁄10 the diameter of a typical bacterium, and able to maintain its structure
when bombarded with electrons under a high vacuum! Such a thin slice cannot be
cut unless the specimen has support of some kind; the necessary support is
provided by plastic. After fixation with chemicals like glutaraldehyde or
osmium tetroxide to stabilize cell structure, the specimen is dehydrated with
organic solvents (e.g., acetone or ethanol). Complete dehydration is essential
because most plastics used for embedding are not water soluble. Next the
specimen is soaked in unpolymerized, liquid epoxy plastic until it is
completely permeated, and then the plastic is hardened to form a solid block.
Thin sections are cut from this block with a glass or diamond knife using a
special instrument called an ultramicrotome.
Cells usually must be stained before they can
be seen clearly in the bright-field microscope; the same is true for
observations with the TEM. The probability of electron scattering is determined
by the density (atomic number) of the specimen atoms. Biological molecules are
composed primarily of atoms with low atomic numbers (H, C, N, and O), and
electron scattering is fairly constant throughout the unstained cell.
Therefore specimens are prepared for
observation by soaking thin sections with solutions of heavy metal salts like
lead citrate and uranyl acetate. The lead and uranium ions bind to cell structures
and make them more electron opaque, thus increasing contrast in the material.
Heavy osmium atoms from the osmium tetroxide fixative also “stain” cells and
increase their contrast. The stained thin sections are then mounted on tiny copper
grids and viewed.
Although specimens normally are embedded in
plastic and thin sectioned to reveal the internal structure of the smallest
cell, there are other ways in which microorganisms and smaller objects can be
readied for viewing. One very useful technique is negative staining. The
specimen is spread out in a thin film with either phosphotungstic acid or
uranyl acetate. Just as in negative staining for light microscopy, heavy metals
do not penetrate the specimen but render the background dark, whereas the
specimen appears bright in photographs. Negative staining is an excellent way
to study the structure of viruses, bacterial gas vacuoles, and other similar
material. A microorganism also can be viewed after shadowing with metal.
It is coated with a thin film of platinum or other heavy metal by evaporation
at an angle of about 45° from horizontal so that the metal strikes the
microorganism on only one side. The area coated with metal scatters electrons
and appears light in photographs, whereas the uncoated side and the shadow region
created by the object is dark (figure 2.24). The specimen looks much as
it would if light were shining on it to cast a shadow.
This technique is particularly useful in
studying virus morphology, bacterial flagella, and plasmids. The TEM will also
disclose the shape of organelles within microorganisms if specimens are
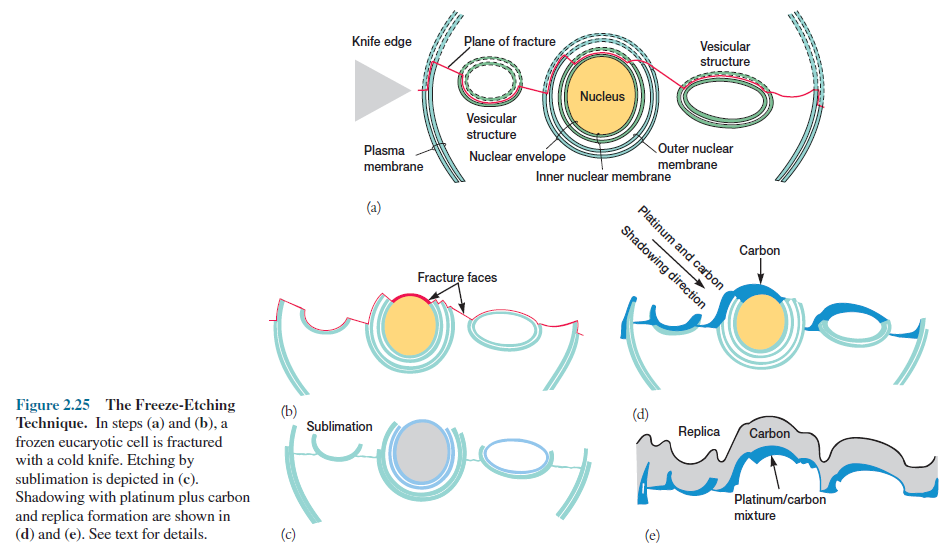
prepared by the freeze-etching procedure.
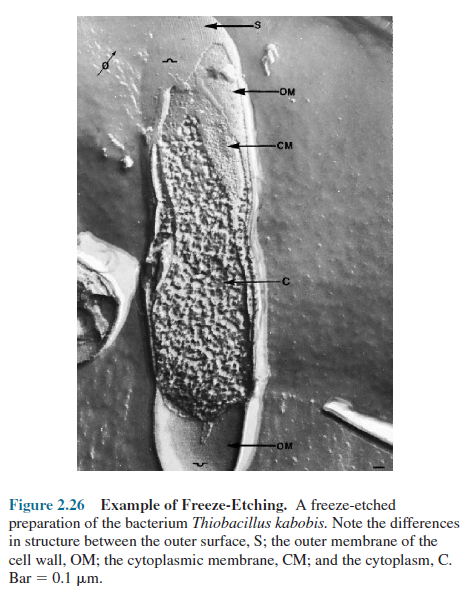
Cells are rapidly frozen in liquid nitrogen and then warmed to -100°C in a vacuum chamber. Next a knife that has been pre-cooled with liquid nitrogen (-196°C) fractures the frozen cells, which are very brittle and break along lines of greatest weakness, usually down the middle of internal membranes (figure 2.25). The specimen is left in the high vacuum for a minute or more so that some of the ice can sublimate away and uncover more structural detail (sometimes this etching step is eliminated). Finally, the exposed surfaces are shadowed and coated with layers of platinum and carbon to form a replica of the surface.
After the specimen has been removed chemically,
this replica is studied in the TEM and provides a detailed, three-dimensional
view of intracellular structure (figure 2.26). An advantage of
freeze-etching is that it minimizes the danger of artifacts because the cells
are frozen quickly rather than being subjected to chemical fixation,
dehydration, and plastic embedding.
The Scanning Electron Microscope
The previously described microscopes form an
image from radiation that has passed through a specimen. More recently the scanning
electron microscope (SEM) has been used
to examine the surfaces of microorganisms in great detail; many instruments have
a resolution of 7 nm or less. The SEM differs from other electron microscopes
in producing an image from electrons emitted by an object’s surface rather than
from transmitted electrons.
Specimen preparation is easy, and in some
cases air-dried material can be examined directly. Most often, however,
microorganisms must first be fixed, dehydrated, and dried to preserve surface
structure and prevent collapse of the cells when they are exposed to the SEM’s
high vacuum. Before viewing, dried samples are mounted and coated with a thin
layer of metal to prevent the buildup of an electrical charge on the surface
and to give a better image.
The SEM scans a narrow, tapered electron beam
back and forth over the specimen (figure 2.27).
When the beam strikes a particular area, surface atoms discharge a tiny shower
of electrons called secondary electrons, and these are trapped by a special
detector.
Secondary electrons entering the detector
strike a scintillator causing it to emit light flashes that a photomultiplier
converts to an electrical current and amplifies. The signal is sent to a cathode-ray
tube and produces an image like a television picture, which can be viewed or
photographed.
The number of secondary electrons reaching
the detector depends on the nature of the specimen’s surface. When the electron
beam strikes a raised area, a large number of secondary electrons enter the
detector; in contrast, fewer electrons escape a depression in the surface and
reach the detector. Thus raised areas appear lighter on the screen and
depressions are darker. A realistic three-dimensional image of the
microorganism’s surface with great depth of focus results (figure 2.28).
The actual in situ location of microorganisms
in ecological niches such as the human skin and the lining of the gut also can
be examined.
2.5 Newer Techniques in Microscopy
Confocal Microscopy
A conventional light microscope, which uses a
mixed wavelength light source and illuminates a large area of the specimen,
will have a relatively great depth of field. Even if not in focus, images of bacteria
from all levels within the field will be visible. These will include cells
above, in, and below the plane of focus (figure 2.29).
As a result the image can be murky, fuzzy,
and crowded. The solution to this problem is the confocal
scanning laser microscope (CSLM) or confocal
microscope. Fluorescently stained specimens are usually examined. A focused
laser beam strikes a point in the specimen (figure 2.30).
Light from the illuminated spot is focused by an objective lens onto a plane
above the objective. An aperture above the objective lens blocks out stray
light from parts of the specimen that lie above and below the plane of focus.
The laser is scanned over a plane in the specimen (beam scanning) or the stage is
moved (stage scanning) and a detector measures the illumination from each point
to produce an image of the optical section. When many optical sections are
scanned, a computer can combine them to form a three-dimensional image from the
digitized signals. This image can be measured and analyzed quantitatively.
The confocal microscope improves images in
two ways.
First, illumination of one spot at a time
reduces interference from light scattering by the rest of the specimen. Second,
the aperture above the objective lens blocks out stray light as previously
mentioned.
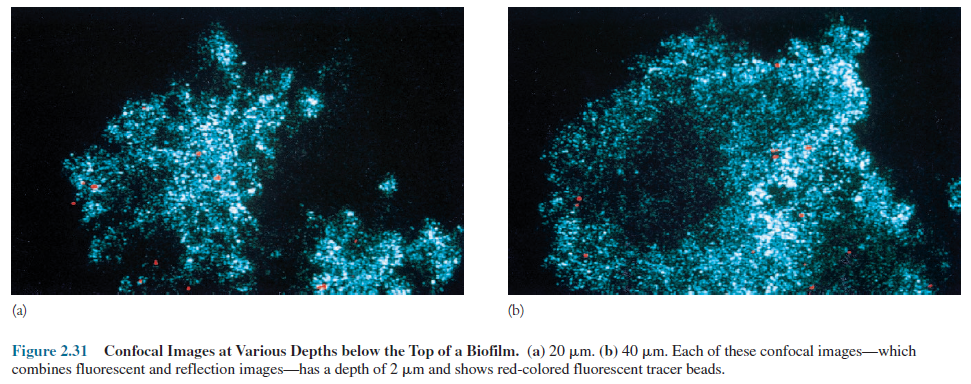
Consequently the image has excellent contrast
and resolution. A depth of 1 _m or less in a thick preparation
can be directly observed. Special computer software is used to create high-resolution,
three-dimensional images of cell structures and complex specimens such as
biofilms (figure
2.31).
Scanning Probe Microscopy
Although light and electron microscopes have become
quite sophisticated and reached an advanced state of development, powerful new
microscopes are still being created. A new class of microscopes, called scanning
probe microscopes, measure surface features by
moving a sharp probe over the object’s surface. The scanning
tunneling microscope, invented in
1980, is an excellent example of a scanning probe microscope. It can achieve
magnifications of 100 million and allow scientists to view atoms on the surface
of a solid. The electrons surrounding surface atoms tunnel or project out from
the surface boundary a very short distance. The scanning tunneling microscope
has a needlelike probe with a point so sharp that often there is only one atom
at its tip. The probe is lowered toward the specimen surface until its electron
cloud just touches that of the surface atoms. If a small voltage is applied
between the tip and specimen, electrons flow through a narrow channel in the
electron clouds. This tunneling current, as it is called, is extraordinarily
sensitive to distance and will decrease about a thousand-fold if the probe is
moved away from the surface by a distance equivalent to the diameter of an
atom.
The arrangement of atoms on the specimen
surface is determined by moving the probe tip back and forth over the surface while
keeping it at a constant height by adjusting the probe distance to maintain a
steady tunneling current. As the tip moves up and down while following the
surface contours, its motion is recorded and analyzed by a computer to create
an accurate three dimensional image of the surface atoms. The surface map can
be displayed on a computer screen or plotted on paper. The resolution is so
great that individual atoms are observed easily. The microscope’s inventors,
Gerd Binnig and Heinrich Rohrer, shared the 1986 Nobel Prize in Physics for their
work, together with Ernst Ruska, the designer of the first transmission
electron microscope.
The scanning tunneling microscope will likely
have a major impact in biology. Recently it has been used to directly view DNA (figure 2.32). Since the microscope can examine objects when they are
immersed in water, it may be particularly useful in studying biological
molecules.
More recently a second type of scanning probe
microscope has been developed. The atomic force microscope moves a sharp probe over the specimen surface while
keeping the distance between the probe tip and the surface constant. It does
this by exerting a very small amount of force on the tip, just enough to
maintain a constant distance but not enough force to damage the surface. The
vertical motion of the tip usually is followed by measuring the deflection of a
laser beam that strikes the lever holding the probe. Unlike the scanning tunneling
microscope, the atomic force microscope can be used to study surfaces that do
not conduct electricity well. The atomic force microscope has been used to
study the interactions between the E. coli GroES
and GroEL chaperonin proteins, to map plasmids by locating restriction enzymes
bound to specific sites, and to follow the behavior of living bacteria and
other cells.



























No comments:
Post a Comment