All microorganisms need access to a source of energy and the raw materials essential for the construction of cellular components. All organisms must have carbon, hydrogen, oxygen, nitrogen, sulfur, phosphorus, and a variety of minerals; many also require one or more special growth factors. The cell takes up these substances by membrane transport processes, the most important of which are facilitated diffusion, active transport, and group translocation. Eucaryotic cells also employ endocytosis.
This
chapter concentrates more directly on the growth. The nature of growth and the
ways in which it can be measured are described first, followed by consideration
of continuous culture techniques. An account of the influence of environmental
factors on microbial growth completes the chapter.
Growth may be defined as an increase in cellular
constituents. It leads to a rise in cell number when microorganisms reproduce
by processes like budding or binary fission. In the latter, individual cells
enlarge and divide to yield two progeny of approximately equal size.
Growth
also results when cells simply become longer or larger. If the microorganism is
coenocytic—that is, a multinucleate organism in which nuclear divisions
are not accompanied by cell divisions—growth results in an increase in cell
size but not cell number. It is usually not convenient to investigate the
growth and reproduction of individual microorganisms because of their small
size. Therefore, when studying growth, microbiologists normally follow changes
in the total population number.
The Bacterial Growth Curve
Population
growth is studied by analyzing the growth curve of a microbial culture. When
microorganisms are cultivated in liquid medium, they usually are grown in a batch
culture or closed system— that is, they are incubated in a closed culture
vessel with a single batch of medium. Because no fresh medium is provided during
incubation, nutrient concentrations decline and concentrations of wastes
increase. The growth of microorganisms reproducing by binary fission can be
plotted as the logarithm of the number of viable cells versus the incubation
time. The resulting curve has four distinct phases (figure 6.1).
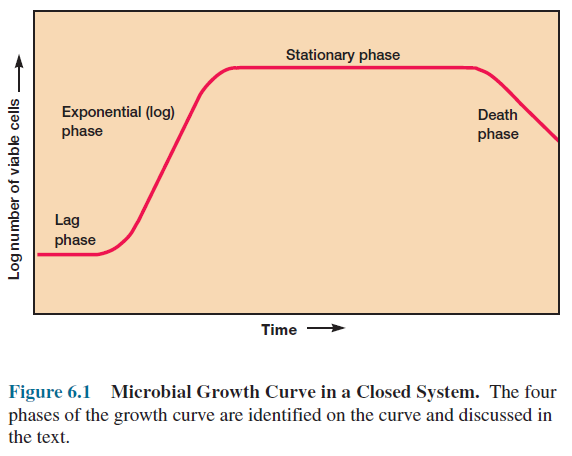 |
Lag Phase
When
microorganisms are introduced into fresh culture medium, usually no immediate
increase in cell number occurs, and therefore this period is called the lag
phase. Although cell division does not take place right away and there is
no net increase in mass, the cell is synthesizing new components. A lag phase
prior to the start of cell division can be necessary for a variety of reasons.
The
cells may be old and depleted of ATP, essential cofactors, and ribosomes; these
must be synthesized before growth can begin. The medium may be different from
the one the microorganism was growing in previously. Here new enzymes would be needed
to use different nutrients. Possibly the microorganisms have been injured and
require time to recover. Whatever the causes, eventually the cells retool,
replicate their DNA, begin to increase in mass, and finally divide.
The
lag phase varies considerably in length with the condition of the
microorganisms and the nature of the medium. This phase may be quite long if
the inoculum is from an old culture or one that has been refrigerated.
Inoculation of a culture into a chemically different medium also results in a
longer lag phase.
Exponential Phase
During
the exponential or log phase, microorganisms are growing and
dividing at the maximal rate possible given their genetic potential, the nature
of the medium, and the conditions under which they are growing. Their rate of
growth is constant during the exponential phase; that is, the microorganisms
are dividing and doubling in number at regular intervals. Because each
individual divides at a slightly different moment, the growth curve rises
smoothly rather than in discrete jumps (figure 6.1). The population is most
uniform in terms of chemical and physiological properties during this phase;
therefore exponential phase cultures are usually used in biochemical and
physiological studies.
Exponential
growth is balanced growth. That is, all cellular constituents are
manufactured at constant rates relative to each other. If nutrient levels or
other environmental conditions change, unbalanced growth results. This
is growth during which the rates of synthesis of cell components vary relative
to one another until a new balanced state is reached. This response is readily
observed in a shift-up experiment in which bacteria are transferred from a
nutritionally poor medium to a richer one. The cells first construct new
ribosomes to enhance their capacity for protein synthesis. This is followed by
increases in protein and DNA synthesis.
Finally,
the expected rise in reproductive rate takes place. Unbalanced growth also
results when a bacterial population is shifted down from a rich medium to a
poor one. The organisms may previously have been able to obtain many cell
components directly from the medium. When shifted to a nutritionally inadequate
medium, they need time to make the enzymes required for the biosynthesis of
unavailable nutrients.
Consequently
cell division and DNA replication continue after the shift-down, but net
protein and RNA synthesis slow. The cells become smaller and reorganize
themselves metabolically until they are able to grow again. Then balanced
growth is resumed and the culture enters the exponential phase.
These
shift-up and shift-down experiments demonstrate that microbial growth is under
precise, coordinated control and responds quickly to changes in environmental
conditions.
When
microbial growth is limited by the low concentration of a required nutrient,
the final net growth or yield of cells increases with the initial amount of the
limiting nutrient present (figure 6.2a). This is the basis of
microbiological assays for vitamins and other growth factors. The rate of
growth also increases with nutrient concentration (figure 6.2b), but in
a hyperbolic manner much like that seen with many enzymes (see figure 8.17).
The
shape of the curve seems to reflect the rate of nutrient uptake by microbial
transport proteins. At sufficiently high nutrient levels the transport systems
are saturated, and the growth rate does not rise further with increasing
nutrient concentration.
Stationary Phase
Eventually
population growth ceases and the growth curve becomes horizontal (figure 6.1).
This stationary phase usually is attained by bacteria at a population
level of around 109 cells per ml. Other microorganisms normally do not reach
such high population densities, protozoan and algal cultures often having
maximum concentrations of about 106 cells per ml. Of course final population
size depends on nutrient availability and other factors, as well as the type of
microorganism being cultured. In the stationary phase the total number of
viable microorganisms remains constant. This may result from a balance between
cell division and cell death, or the population may simply cease to divide
though remaining metabolically active.
Microbial
populations enter the stationary phase for several reasons. One obvious factor
is nutrient limitation; if an essential nutrient is severely depleted,
population growth will slow. Aerobic organisms often are limited by O2
availability. Oxygen is not very soluble and may be depleted so quickly that
only the surface of a culture will have an O2 concentration adequate
for growth.
The
cells beneath the surface will not be able to grow unless the culture is shaken
or aerated in another way. Population growth also may cease due to the
accumulation of toxic waste products.
This factor
seems to limit the growth of many anaerobic cultures (cultures growing in the
absence of O2). For example, streptococci can produce so much lactic
acid and other organic acids from sugar fermentation that their medium becomes
acidic and growth is inhibited. Streptococcal cultures also can enter the
stationary phase due to depletion of their sugar supply. Finally, there is some
evidence that growth may cease when a critical population level is reached.
Thus entrance into the stationary phase may result from several factors
operating in concert.
As we
have seen, bacteria in a batch culture may enter stationary phase in response
to starvation. This probably often occurs in nature as well because many
environments have quite low nutrient levels.
Starvation
can be a positive experience for bacteria. Many do not respond with obvious
morphological changes such as endospore formation, but only decrease somewhat
in overall size, often accompanied by protoplast shrinkage and nucleoid
condensation. The more important changes are in gene expression and physiology.
Starving
bacteria frequently produce a variety of starvation proteins, which make
the cell much more resistant to damage in a variety of ways. They
increase peptidoglycan cross-linking and cell wall strength. The Dps (DNA-binding protein from starved
cells) protein protects DNA. Chaperones prevent protein denaturation and renature
damaged proteins. As a result of these and many other mechanisms, the starved
cells become harder to kill and more resistant to starvation itself, damaging
temperature changes, oxidative and osmotic damage, and toxic chemicals such as
chlorine.
These
changes are so effective that some bacteria can survive starvation for years.
Clearly, these considerations are of great practical importance in medical and
industrial microbiology. There is even evidence that Salmonella typhimurium and
some other bacterial pathogens become more virulent when starved.
Death Phase or Phase of Decline
Detrimental
environmental changes like nutrient deprivation and the buildup of toxic wastes
lead to the decline in the number of viable cells characteristic of the death
phase. The death of a microbial population, like its growth during the
exponential phase, is usually logarithmic (that is, a constant proportion of
cells dies every hour). This pattern in viable cell count holds even when the total
cell number remains constant because the cells simply fail to lyse after dying.
Often the only way of deciding whether a bacterial cell is viable is by
incubating it in fresh medium; if it does not grow and reproduce, it is assumed
to be dead. That is, death is defined to be the irreversible loss of the
ability to reproduce.
Although
most of a microbial population usually dies in a logarithmic fashion, the death
rate may decrease after the population has been drastically reduced. This is
due to the extended survival of particularly resistant cells. For this and
other reasons, the death phase curve may be complex.
The Mathematics
of Microbial Growth
Knowledge
of microbial growth rates during the exponential phase is indispensable to
microbiologists. Growth rate studies contribute to basic physiological and
ecological research and the solution of applied problems in industry. Therefore
the quantitative aspects of exponential phase growth will be discussed.
During
the exponential phase each microorganism is dividing at constant intervals.
Thus the population will double in number during a specific length of time
called the generation time or doubling time. This situation can be illustrated
with a simple example.
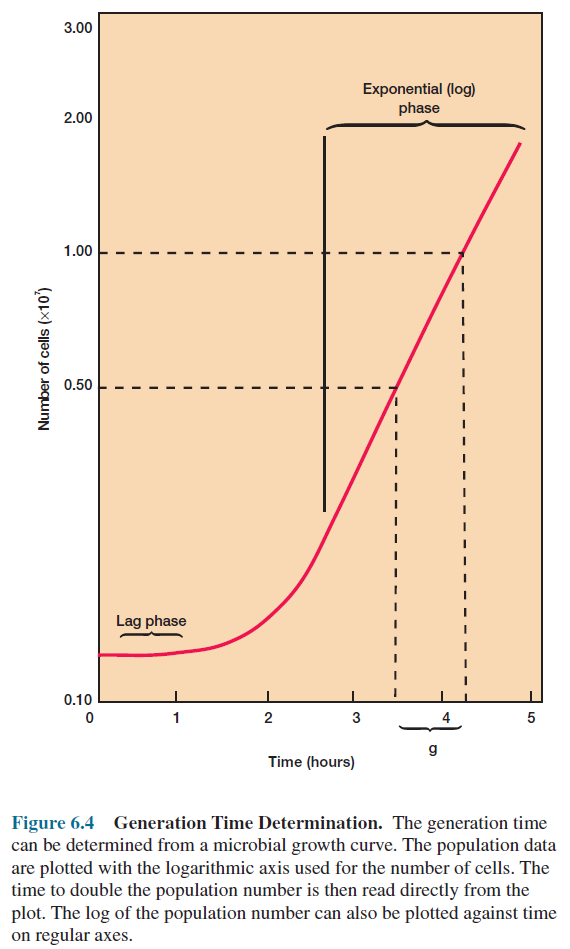
Suppose that a culture tube is inoculated with one cell that divides every 20 minutes (table 6.1). The population will be 2 cells after 20 minutes, 4 cells after 40 minutes, and so forth. Because the population is doubling every generation, the increase in population is always 2n where n is the number of generations. The resulting population increase is exponential or logarithmic (figure 6.3).
Measurement
of Microbial Growth
There
are many ways to measure microbial growth to determine growth rates and
generation times. Either population mass or number may be followed because
growth leads to increases in both. The most commonly employed techniques for growth
measurement are examined briefly and the advantages and disadvantages of each
noted. No single technique is always best; the most appropriate approach will
depend on the experimental situation.
Measurement
of Microbial Cell Numbers
The
most obvious way to determine microbial numbers is through direct counting.
Using a counting chamber is easy, inexpensive, and relatively quick; it also
gives information about the size and morphology of microorganisms.
Petroff-Hausser counting chambers can be used for counting procaryotes;
hemocytometers can be used for both procaryotes and eucaryotes.
Procaryotes
are more easily counted in these chambers if they are stained, or when a
phase-contrast or a fluorescence microscope is employed. These specially designed
slides have chambers of known depth with an etched grid on the chamber bottom (figure
6.5). The number of microorganisms in a sample can be calculated by taking into
account the chamber’s volume and any sample dilutions required. There are some
disadvantages to the technique. The microbial population must be fairly large
for accuracy because such a small volume is sampled. It is also difficult to
distinguish between living and dead cells in counting chambers without special
techniques.
Larger
microorganisms such as protozoa, algae, and nonfilamentous yeasts can be
directly counted with electronic counters such as the Coulter Counter. The
microbial suspension is forced through a small hole or orifice. An electrical
current flows through the hole, and electrodes placed on both sides of the
orifice measure its electrical resistance. Every time a microbial cell passes
through the orifice, electrical resistance increases (or the conductivity
drops) and the cell is counted. The Coulter Counter gives accurate results with
larger cells and is extensively used in hospital laboratories to count red and
white blood cells. It is not as useful in counting bacteria because of interference
by small debris particles, the formation of filaments, and other problems.
Counting
chambers and electronic counters yield counts of all cells, whether alive or
dead. There are also several viable counting techniques, procedures specific
for cells able to grow and reproduce. In most viable counting procedures, a
diluted sample of bacteria or other microorganisms is dispersed over a solid
agar surface. Each microorganism or group of microorganisms develops into a
distinct colony. The original number of viable microorganisms in the sample can
be calculated from the number of colonies formed and the sample dilution. For
example, if 1.0 ml of a 1X10-6 dilution yielded 150 colonies, the
original sample contained around 1.5X 108 cells per ml. Usually the count
is made more accurate by use of a special colony counter.
In
this way the spread-plate and pour-plate techniques may be used to find the
number of microorganisms in a sample.
Plating
techniques are simple, sensitive, and widely used for viable counts of bacteria
and other microorganisms in samples of food, water, and soil. Several problems,
however, can lead to inaccurate counts. Low counts will result if clumps of
cells are not broken up and the microorganisms well dispersed. Because it is
not possible to be absolutely certain that each colony arose from an individual
cell, the results are often expressed in terms of colony forming units (CFU)
rather than the number of microorganisms.
The
samples should yield between 30 and 300 colonies for best results. Of course
the counts will also be low if the agar medium employed cannot support growth
of all the viable microorganisms present.
The
hot agar used in the pour-plate technique may injure or kill sensitive cells;
thus spread plates sometimes give higher counts than pour plates.
Microbial
numbers are frequently determined from counts of colonies growing on special
membrane filters having pores small enough to trap bacteria. In the membrane
filter technique, a sample is drawn through a special membrane filter (figure 6.6).
The filter is then placed on an agar medium or on a pad soaked with liquid
media and incubated until each cell forms a separate colony. A colony count
gives the number of microorganisms in the filtered sample, and special media
can be used to select for specific microorganisms (figure 6.7). This technique
is especially useful in analyzing aquatic samples.
The
bacteria then are stained with a fluorescent dye such as acridine orange or
DAPI and observed microscopically. Acridine orange–stained microorganisms glow
orange or green and are easily counted with an epifluorescence microscope.
Usually the counts obtained with this approach are much higher than those from
culture techniques because some of the bacteria are dead. Commercial kits that
use fluorescent reagents to stain live and dead cells differently are now
available. This makes it possible to directly count the number of live and dead
microorganisms in a sample (see figure 2.13d).
Measurement
of Microbial Cell Mass
Increases
in the total cell mass, as well as in cell numbers, accompany population
growth. Therefore techniques for measuring changes in cell mass can be used in
following growth. The most direct approach is the determination of microbial
dry weight.
Cells
growing in liquid medium are collected by centrifugation, washed, dried in an
oven, and weighed. This is an especially useful technique for measuring the
growth of fungi. It is time consuming, however, and not very sensitive. Because
bacteria weigh so little, it may be necessary to centrifuge several hundred
milliliters of culture to collect a sufficient quantity.
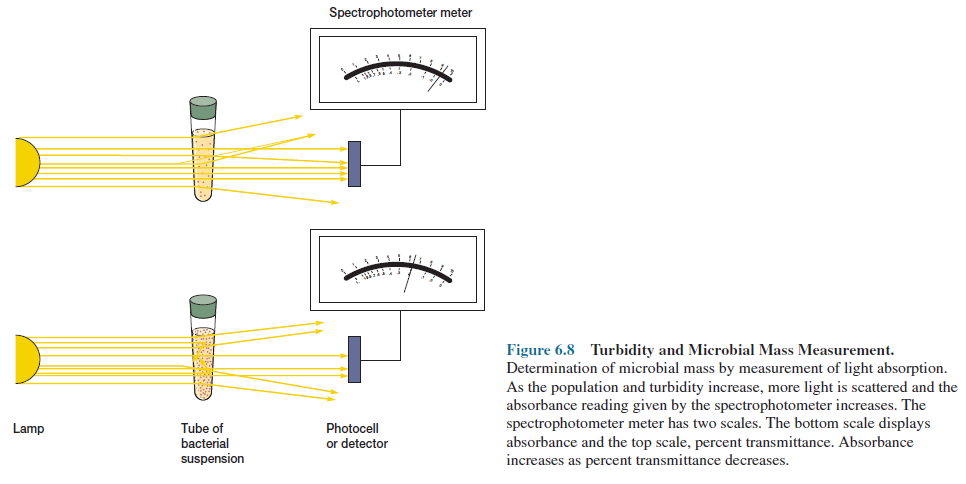
More
rapid, sensitive techniques depend on the fact that microbial cells scatter
light striking them. Because microbial cells in a population are of roughly
constant size, the amount of scattering is directly proportional to the biomass
of cells present and indirectly related to cell number. When the concentration
of bacteria reaches about 10 million cells (107) per ml, the medium appears slightly
cloudy or turbid.
Further
increases in concentration result in greater turbidity and less light is
transmitted through the medium. The extent of light scattering can be measured
by a spectrophotometer and is almost linearly related to bacterial
concentration at low absorbance levels (figure 6.8). Thus population growth can
be easily measured spectrophotometrically as long as the population is high
enough to give detectable turbidity.
If
the amount of a substance in each cell is constant, the total quantity of that
cell constituent is directly related to the total microbial cell mass. For
example, a sample of washed cells collected from a known volume of medium can
be analyzed for total protein or nitrogen. An increase in the microbial
population will be reflected in higher total protein levels. Similarly,
chlorophyll determinations can be used to measure algal populations, and the
quantity of ATP can be used to estimate the amount of living microbial mass.
The
Continuous Culture of Microorganisms
Up to
this point the focus has been on closed systems called batch cultures in which
nutrient supplies are not renewed nor wastes removed.
Exponential
growth lasts for only a few generations and soon the stationary phase is
reached. However, it is possible to grow microorganisms in an open system, a
system with constant environmental conditions maintained through continual
provision of nutrients and removal of wastes.
These
conditions are met in the laboratory by a continuous culture system. A
microbial population can be maintained in the exponential growth phase and at a
constant biomass concentration for extended periods in a continuous culture
system.
The Chemostat Continuous Culture System
Two major
types of continuous culture systems commonly are used: (1) chemostats and (2)
turbidostats.
A
chemostat is constructed so that sterile medium is fed into the culture vessel
at the same rate as the media containing microorganisms is removed (figure 6.9).
The culture medium for a chemostat possesses an essential nutrient (e.g., an
amino acid) in limiting quantities. Because of the presence of a limiting
nutrient, the growth rate is determined by the rate at which new medium is fed
into the growth chamber, and the final cell density depends on the
concentration of the limiting nutrient.
The
rate of nutrient exchange is expressed as the dilution rate (D), the
rate at which medium flows through the culture vessel relative to the vessel
volume, where f is the flow rate (ml/hr) and V is the vessel
volume (ml).
D = f/V
For
example, if f is 30 ml/hr and V is 100 ml, the dilution rate is 0.30
hr-1 .
Both
the microbial population level and the generation time are related to the
dilution rate (figure 6.10). The microbial population density remains unchanged
over a wide range of dilution rates. The generation time decreases (i.e., the
growth rate rises) as the dilution rate increases.
The
limiting nutrient will be almost completely depleted under these balanced conditions.
If the dilution rate rises too high, the microorganisms can actually be washed
out of the culture vessel before reproducing because the dilution rate is
greater than the maximum growth rate. The limiting nutrient concentration rises
at higher dilution rates because fewer microorganisms are present to use it.
At
very low dilution rates, an increase in D causes a rise in both cell
density and the growth rate. This is because of the effect of nutrient
concentration on the growth rate, sometimes called the Monod relationship
(figure 6.2b). Only a limited supply of nutrient is available at low
dilution rates. Much of the available energy must be used for cell maintenance,
not for growth and reproduction.
As
the dilution rate increases, the amount of nutrients and the resulting cell
density rise because energy is available for both maintenance and growth. The
growth rate increases when the total available energy exceeds the maintenance
energy.
The
Turbidostat
The
second type of continuous culture system, the turbidostat, has a photocell that
measures the absorbance or turbidity of the culture in the growth vessel. The
flow rate of media through the vessel is automatically regulated to maintain a
predetermined turbidity or cell density. The turbidostat differs from the
chemostat in several ways. The dilution rate in a turbidostat varies rather than
remaining constant, and its culture medium lacks a limiting nutrient. The
turbidostat operates best at high dilution rates; the chemostat is most stable
and effective at lower dilution rates.
Continuous
culture systems are very useful because they provide a constant supply of cells
in exponential phase and growing at a known rate. They make possible the study
of microbial growth at very low nutrient levels, concentrations close to those present
in natural environments.
These
systems are essential for research in many areas—for example, in studies on
interactions between microbial species under environmental conditions resembling
those in a freshwater lake or pond. Continuous systems also are used in food
and industrial microbiology.
The Influence
of Environmental Factors on Microbial Growth
Microorganisms
must be able to respond to variations in nutrient levels, and particularly to
nutrient limitation. The growth of microorganisms also is greatly affected by
the chemical and physical nature of their surroundings.
An
understanding of environmental influences aids in the control of microbial
growth and the study of the ecological distribution of microorganisms.
The
ability of some microorganisms to adapt to extreme and inhospitable
environments is truly remarkable. Procaryotes are present anywhere life can
exist. Many habitats in which prokaryotes thrive would kill most other organisms.
Procaryotes such as Bacillus infernus even seem able to live over 1.5
miles below the Earth’s surface, without oxygen and at temperatures above 60°C.
Microorganisms
that grow in such harsh conditions are often called extremophiles.
In
this section we shall briefly review how some of the most important
environmental factors affect microbial growth. Major emphasis will be given to
solutes and water activity, pH, temperature, oxygen level, pressure, and
radiation. Table 6.3 summarizes the way in which microorganisms are categorized
in terms of their response to these factors.
The Influence of Solutes and
Water Activity on Microbial Growth
Because
a selectively permeable plasma membrane separates microorganisms from their
environment, they can be affected by changes in the osmotic concentration of
their surroundings. If a microorganism is placed in a hypotonic solution (one
with a lower osmotic concentration), water will enter the cell and cause it to
burst unless something is done to prevent the influx. The osmotic concentration
of the cytoplasm can be reduced by use of inclusion bodies. Procaryotes also
can contain pressure-sensitive channels that open to allow solute escape when
the osmolarity of the environment becomes much lower than that of the
cytoplasm.
Most
bacteria, algae, and fungi have rigid cell walls that maintain the shape and
integrity of the cell. When microorganisms with rigid cell walls are placed in
a hypertonic environment, water leaves and the plasma membrane shrinks away
from the wall, a process known as plasmolysis. This dehydrates the cell and may
damage the plasma membrane; the cell usually becomes metabolically inactive and
ceases to grow.
Many
microorganisms keep the osmotic concentration of their protoplasm somewhat
above that of the habitat by the use of compatible solutes, so that the plasma
membrane is always pressed firmly against their cell wall. Compatible solutes
are solutes that are compatible with metabolism and growth when at high
intracellular concentrations.
Most procaryotes increase their internal osmotic concentration in a hypertonic environment through the synthesis or uptake of choline, betaine, proline, glutamic acid, and other amino acids; elevated levels of potassium ions are also involved to some extent. Algae and fungi employ sucrose and polyols—for example, arabitol, glycerol, and mannitol— for the same purpose. Polyols and amino acids are ideal solutes for this function because they normally do not disrupt enzyme structure and function.
A few procaryotes like Halobacterium salinarium
raise their osmotic concentration with potassium ions (sodium ions are also
elevated but not as much as potassium). Halobacterium’s enzymes have
been altered so that they actually require high salt concentrations for normal
activity. Since protozoa do not have a cell wall, they must use contractile
vacuoles to eliminate excess water when living in
hypotonic environments.
The amount of water available to microorganisms can be reduced by interaction with solute molecules (the osmotic effect) or by adsorption to the surfaces of solids (the matric effect). Because the osmotic concentration of a habitat has such profound effects on microorganisms, it is useful to be able to express quantitatively the degree of water availability.
Microbiologists generally use
water activity (aw) for this purpose (water availability also may be expressed
as water potential, which is related to aw). The water activity of a solution
is 1/100 the relative humidity of the solution (when expressed as a percent).
It is also equivalent to the ratio of the solution’s vapor pressure (Psoln)
to that of pure water (Pwater).
Aw=
Psoln/Pwater
The
water activity of a solution or solid can be determined by sealing it in a
chamber and measuring the relative humidity after the system has come to
equilibrium. Suppose after a sample is treated in this way, the air above it is
95% saturated—that is, the air contains 95% of the moisture it would have when
equilibrated at the same temperature with a sample of pure water. The relative humidity
would be 95% and the sample’s water activity, 0.95.
Water
activity is inversely related to osmotic pressure; if a solution has high
osmotic pressure, its aw is low.
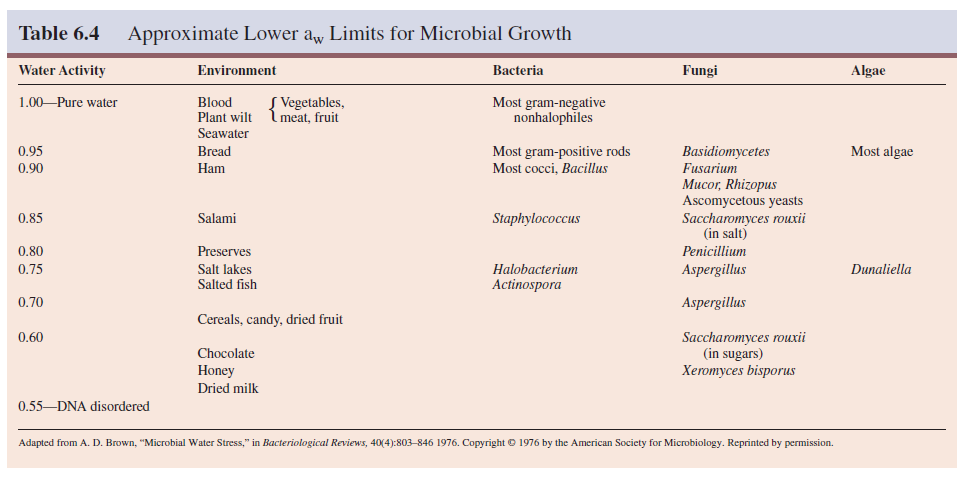
Microorganisms differ greatly in their ability to adapt to habitats with low water activity (table 6.4). A microorganism must expend extra effort to grow in a habitat with a low aw value because it must maintain a high internal solute concentration to retain water. Some microorganisms can do this and are osmotolerant; they will grow over wide ranges of water activity or osmotic concentration.
For example, Staphylococcus
aureus can be cultured in media containing any sodium chloride
concentration up to about 3 M. It is well adapted for growth on the skin. The yeast
Saccharomyces rouxii will grow in sugar solutions with aw values as low
as 0.6. The alga Dunaliella viridis tolerates sodium chloride
concentrations from 1.7 M to a saturated solution.
Although
a few microorganisms are truly osmotolerant, most only grow well at water
activities around 0.98 (the approximate aw for seawater) or higher. This is why
drying food or adding large quantities of salt and sugar is so effective in
preventing food spoilage. As table 6.4 shows, many fungi are osmotolerant and thus
particularly important in the spoilage of salted or dried foods.
Halophiles have adapted so completely to hypertonic, saline conditions that they require high levels of sodium chloride to grow, concentrations between about 2.8 M and saturation (about 6.2 M) for extreme halophilic bacteria. The archaeon Halobacterium can be isolated from the Dead Sea (a salt lake between Israel and Jordan and the lowest lake in the world), the Great Salt Lake in Utah, and other aquatic habitats with salt concentrations approaching saturation.
Halobacterium and other extremely halophilic bacteria have significantly modified the structure of their proteins and membranes rather than simply increasing the intracellular concentrations of solutes, the approach used by most osmotolerant microorganisms. These extreme halophiles accumulate enormous quantities of potassium in order to remain hypertonic to their environment; the internal potassium concentration may reach 4 to 7 M. The enzymes, ribosomes, and transport proteins of these bacteria require high levels of potassium for stability and activity.
In addition, the plasma membrane and cell wall of Halobacterium
are stabilized by high concentrations of sodium ion. If the sodium
concentration decreases too much, the wall and plasma membrane literally
disintegrate. Extreme halophilic bacteria have successfully adapted to
environmental conditions that would destroy most organisms. In the process they
have become so specialized that they have lost ecological flexibility and can
prosper only in a few extreme habitats.
The Influence of pH on Microbial Growth
pH is
a measure of the hydrogen ion activity of a solution and is defined as the
negative logarithm of the hydrogen ion concentration (expressed in terms of
molarity).
pH = -log [H+]
= log(1/[H+])
The
pH scale extends from pH 0.0 (1.0 M H+) to pH 14.0 (1.0 X 10-14
M H+), and each pH unit represents a tenfold change in hydrogen ion
concentration. Figure 6.11 shows that the habitats in which microorganisms grow
vary widely—from pH 1 to 2 at the acid end to alkaline lakes and soil that may
have pH values between 9 and 10.
It is not surprising that pH dramatically affects microbial growth. Each species has a definite pH growth range and pH growth optimum. Acidophiles have their growth optimum between pH 0 and 5.5; neutrophiles, between pH 5.5 and 8.0; and alkalophiles prefer the pH range of 8.5 to 11.5. Extreme alkalophiles have growth optima at pH 10 or higher. In general, different microbial groups have characteristic pH preferences.
Most bacteria and protozoa are neutrophiles. Most fungi prefer
slightly acid surroundings, about pH 4 to 6; algae also seem to favor slight acidity.
There are many exceptions to these generalizations. For example, the alga Cyanidium
caldarium and the archaeon Sulfolobus acidocaldarius are common
inhabitants of acidic hot springs; both grow well around pH 1 to 3 and at high
temperatures.
The
Archaea Ferroplasma acidarmanus and Picrophilus oshimae can
actually grow at pH 0, or very close to it.
Although
microorganisms will often grow over wide ranges of pH and far from their
optima, there are limits to their tolerance.
Drastic
variations in cytoplasmic pH can harm microorganisms by disrupting the plasma
membrane or inhibiting the activity of enzymes and membrane transport proteins.
Procaryotes die if the internal pH drops much below 5.0 to 5.5. Changes in the
external pH also might alter the ionization of nutrient molecules and thus reduce
their availability to the organism.
Several
mechanisms for the maintenance of a neutral cytoplasmic pH have been proposed.
The plasma membrane may be relatively impermeable to protons. Neutrophiles
appear to exchange potassium for protons using an antiport transport system.
Extreme alkalophiles like Bacillus alcalophilus maintain their internal
pH closer to neutrality by exchanging internal sodium ions for external
protons. Internal buffering also may contribute to pH homeostasis.
Microorganisms often must adapt to environmental pH changes to survive. In bacteria, potassium/proton and sodium/proton antiport systems probably correct small variations in pH. If the pH becomes too acidic, other mechanisms come into play. When the pH drops below about 5.5 to 6.0, Salmonella typhimurium and E. coli synthesize an array of new proteins as part of what has been called their acidic tolerance response.
A proton-translocating ATPase contributes to this protective
response, either by making more ATP or by pumping protons out of the cell. If
the external pH decreases to 4.5 or lower, chaperones such as acid shock
proteins and heat shock proteins are synthesized. Presumably these prevent the
acid denaturation of proteins and aid in the refolding of denatured proteins.
Microorganisms
frequently change the pH of their own habitat by producing acidic or basic
metabolic waste products. Fermentative microorganisms form organic acids from
carbohydrates, whereas chemolithotrophs like Thiobacillus oxidize reduced
sulfur components to sulfuric acid. Other microorganisms make their environment
more alkaline by generating ammonia through amino acid degradation.
Buffers
often are included in media to prevent growth inhibition by large pH changes.
Phosphate is a commonly used buffer and a good example of buffering by a weak
acid (H2PO4–) and its conjugate base (HPO4 2–).
If
protons are added to the mixture, they combine with the salt form to yield a
weak acid. An increase in alkalinity is resisted because the weak acid will
neutralize hydroxyl ions through proton donation to give water. Peptides and
amino acids in complex media also have a strong buffering effect.
The Influence of Temperature on Microbial Growth
Environmental
temperature profoundly affects microorganisms, like all other organisms.
Indeed, microorganisms are particularly susceptible because they are usually
unicellular and their temperature varies with that of the external environment.
For these reasons, microbial cell temperature directly reflects that of the
cell’s surroundings. A most important factor influencing the effect of temperature
on growth is the temperature sensitivity of enzyme catalyzed reactions.
At
low temperatures a temperature rise increases the growth rate because the
velocity of an enzyme-catalyzed reaction, like that of any chemical reaction,
will roughly double for every 10°C rise in temperature. Because the rate of each
reaction increases, metabolism as a whole is more active at higher
temperatures, and the microorganism grows faster.
Beyond
a certain point further increases actually slow growth, and sufficiently high
temperatures are lethal. High temperatures damage microorganisms by denaturing
enzymes, transport carriers, and other proteins. Microbial membranes are also
disrupted by temperature extremes; the lipid bilayer simply melts and
disintegrates.
Thus,
although functional enzymes operate more rapidly at higher temperatures, the
microorganism may be damaged to such an extent that growth is inhibited because
the damage cannot be repaired. At very low temperatures, membranes solidify and
enzymes don’t work rapidly. In summary, when organisms are above the optimum temperature,
both function and cell structures are affected. If temperatures are very low,
function is affected but not necessarily cell chemical composition and
structure.
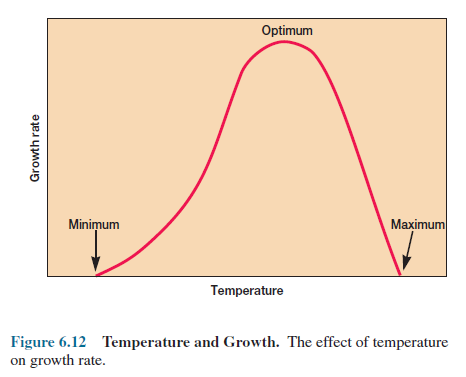
Because
of these opposing temperature influences, microbial growth has fairly characteristic
temperature dependence with distinct cardinal temperatures—minimum, optimum,
and maximum growth temperatures (figure 6.12). Although the shape of the
temperature dependence curve can vary, the temperature optimum is always closer
to the maximum than to the minimum.
The
cardinal temperatures for a particular species are not rigidly fixed but often
depend to some extent on other environmental factors such as pH and the
available nutrients. For example, Crithidia fasciculata, a flagellated
protozoan living in the gut of mosquitos, will grow in a simple medium at 22 to
27°C. However, it cannot be cultured at 33 to 34°C without the addition of
extra metals, amino acids, vitamins, and lipids.
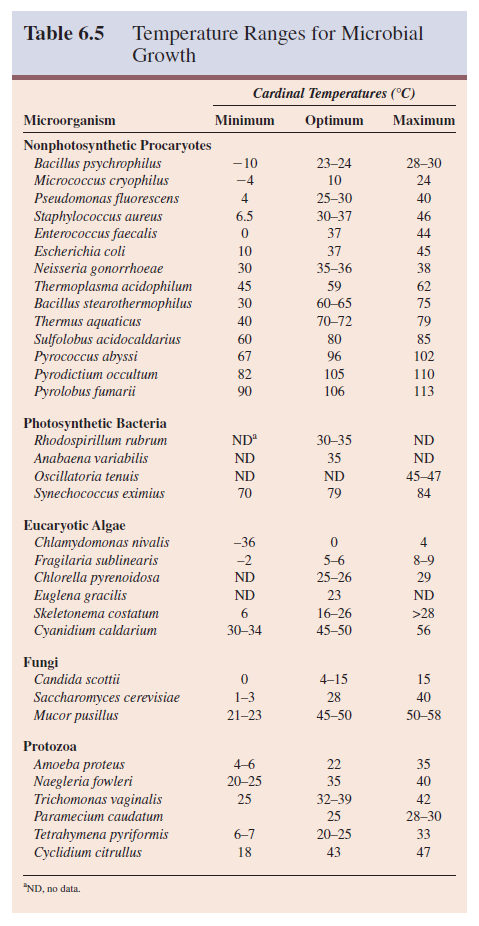
The cardinal temperatures vary greatly between microorganisms (table 6.5). Optima normally range from 0°C to as high as 75°C, whereas microbial growth occurs at temperatures extending from -20°C to over 100°C. The major factor determining this growth range seems to be water. Even at the most extreme temperatures, microorganisms need liquid water to grow.
The growth temperature range for a particular
microorganism usually spans about 30 degrees. Some species (e.g., Neisseria
gonorrhoeae) have a small range; others, like Enterococcus faecalis, will
grow over a wide range of temperatures. The major microbial groups differ from
one another regarding their maximum growth temperature.
The
upper limit for protozoa is around 50°C. Some algae and fungi can grow at
temperatures as high as 55 to 60°C. Procaryotes have been found growing at or
close to 100°C, the boiling point of water at sea level (see figure 20.8).
Recently strains growing at even higher temperatures have been discovered (Box
6.1). Clearly, prokaryotic organisms can grow at much higher temperatures than
eucaryotes.
It
has been suggested that eucaryotes are not able to manufacture organellar
membranes that are stable and functional at temperatures above 60°C. The
photosynthetic apparatus also appears to be relatively unstable because
photosynthetic organisms are not found growing at very high temperatures.
Microorganisms
such as those in table 6.5 can be placed in one of five classes based on
their temperature ranges for growth (figure 6.13).
1. Psychrophiles grow well at 0°C and have an optimum growth temperature of 15°C or lower; the maximum is around 20°C. They are readily isolated from Arctic and Antarctic habitats; because 90% of the ocean is 5°C or colder, it constitutes an enormous habitat for psychrophiles. The psychrophilic alga Chlamydomonas nivalis can actually turn a snowfield or glacier pink with its bright red spores.
Psychrophiles are widespread among bacterial taxa and found in such genera as Pseudomonas, Vibrio, Alcaligenes, Bacillus, Arthrobacter, Moritella, Photobacterium, and Shewanella. The psychrophilic archaeon Methanogenium has recently been isolated from Ace Lake in Antarctica. Psychrophilic microorganisms have adapted to their environment in several ways. Their enzymes, transport systems, and protein synthetic mechanisms function well at low temperatures.
The cell
membranes of psychrophilic microorganisms have high levels of unsaturated fatty
acids and remain semifluid when cold. Indeed, many psychrophiles begin to leak
cellular constituents at temperatures higher than 20°C because of cell membrane
disruption.
2.
Many species can grow at 0 to 7°C even though they have optima between 20 and 30°C,
and maxima at about 35°C. These are called psychrotrophs or facultative
psychrophiles. Psychrotrophic bacteria and fungi are major factors in the spoilage
of refrigerated foods.
3. Mesophiles
are microorganisms with growth optima around 20 to 45°C; they often have a
temperature minimum of 15 to 20°C. Their maximum is about 45°C or lower. Most
microorganisms probably fall within this category. Almost all human pathogens
are mesophiles, as might be expected since their environment is a fairly constant
37°C.
4. Some microorganisms are thermophiles; they can grow at temperatures of 55°C or higher. Their growth minimum is usually around 45°C and they often have optima between 55 and 65°C. The vast majority are procaryotes although a few algae and fungi are thermophilic (table 6.5). These organisms flourish in many habitats including composts, self-heating hay stacks, hot water lines, and hot springs.
Thermophiles differ from mesophiles in having much more heat-stable enzymes
and protein synthesis systems able to function at high temperatures. Their
membrane lipids are also more saturated than those of mesophiles and have
higher melting points; therefore thermophile membranes remain intact at higher
temperatures.
5. As
mentioned previously, a few thermophiles can grow at 90°C or above and some
have maxima above 100°C.
Procaryotes
that have growth optima between 80°C and about 113°C are called hyperthermophiles.
They usually do not grow well below 55°C. Pyrococcus abyssi and Pyrodictium
occultum are examples of marine hyperthermophiles found in hot areas of the
seafloor.
The Influence of Oxygen Concentration on Microbial Growth
An organism able to grow in the presence of atmospheric O2 is an aerobe, whereas one that can grow in its absence is an anaerobe.
Almost
all multicellular organisms are completely dependent on atmospheric O2
for growth—that is, they are obligate aerobes (table 6.3). Oxygen serves
as the terminal electron acceptor for the electron- transport chain in aerobic
respiration. In addition, aerobic eucaryotes employ O2 in the
synthesis of sterols and unsaturated fatty acids. Facultative anaerobes do
not require O2 for growth but do grow better in its presence. In the
presence of oxygen they will use aerobic respiration.
Aerotolerant anaerobes such as Enterococcus faecalis simply
ignore O2 and grow equally well whether it is present or not. In
contrast, strict or obligate anaerobes (e.g., Bacteroides, Fusobacterium,
Clostridium pasteurianum, Methanococcus) do not tolerate O2 at
all and die in its presence. Aerotolerant and strict anaerobes cannot generate
energy through respiration and must employ fermentation or anaerobic
respiration pathways for this purpose.
Finally,
there are aerobes such as Campylobacter called microaerophiles, that
are damaged by the normal atmospheric level of O2 (20%) and require
O2 levels below the range of 2 to 10% for growth. The nature of
bacterial O2 responses can be readily determined by growing the
bacteria in culture tubes filled with a solid culture medium or a special
medium like thioglycollate broth, which contains a reducing agent to lower O2
levels (figure 6.14).
A
microbial group may show more than one type of relationship to O2. All five types
are found among the procaryotes and protozoa.
Fungi
are normally aerobic, but a number of species—particularly among the yeasts—are
facultative anaerobes. Algae are almost always obligate aerobes. It should be
noted that the ability to grow in both aerobic and anaerobic environments
provides considerable flexibility and is an ecological advantage.
Although
strict anaerobes are killed by O2, they may be recovered from
habitats that appear to be aerobic. In such cases they associate with
facultative anaerobes that use up the available O2 and thus make the
growth of strict anaerobes possible. For example, the strict anaerobe Bacteroides
gingivalis lives in the mouth where it grows in the anaerobic crevices
around the teeth.
These
different relationships with O2 appear due to several factors, including the
inactivation of proteins and the effect of toxic O2 derivatives. Enzymes can be
inactivated when sensitive groups like sulfhydryls are oxidized. A notable
example is the nitrogen- fixation enzyme nitrogenase, which is very oxygen
sensitive.
Oxygen
accepts electrons and is readily reduced because its two outer orbital
electrons are unpaired. Flavoproteins, several other cell constituents, and
radiation promote oxygen reduction. The result is usually some combination of
the reduction products superoxide radical, hydrogen peroxide, and hydroxyl
radical.
O2
+ e- → O2.– (superoxide radical)
O2.–
+ e- + 2H+ →H2O2 (hydrogen
peroxide)
H2O2
+ e- + H+ →H2O + OH- (hydroxyl
radical)
These
products of oxygen reduction are extremely toxic because they are powerful
oxidizing agents and rapidly destroy cellular constituents. A microorganism
must be able to protect itself against such oxygen products or it will be
killed. Neutrophils and macrophages use these toxic oxygen products to destroy
invading pathogens.
Many
microorganisms possess enzymes that afford protection against toxic O2
products. Obligate aerobes and facultative anaerobes usually contain the
enzymes superoxide dismutase (SOD) and catalase, which catalyze
the destruction of superoxide radical and hydrogen peroxide, respectively.
Peroxidase also can be used to destroy hydrogen peroxide.
Aerotolerant
microorganisms may lack catalase but almost always have superoxide dismutase.
The aerotolerant Lactobacillus plantarum uses manganous ions instead of
superoxide dismutase to destroy the superoxide radical. All strict anaerobes
lack both enzymes or have them in very low concentrations and therefore cannot
tolerate O2.
Because
aerobes need O2 and anaerobes are killed by it, radically different
approaches must be used when growing the two types of microorganisms. When
large volumes of aerobic microorganisms are cultured, either the culture vessel
is shaken to aerate the medium or sterile air must be pumped through the
culture vessel. Precisely the opposite problem arises with anaerobes; all O2
must be excluded. This can be accomplished in several ways.
(1)
Special anaerobic media containing reducing agents such as thioglycollate or
cysteine may be used. The medium is boiled during preparation to dissolve its
components; boiling also drives off oxygen very effectively. The reducing agents
will eliminate any dissolved O2 remaining within the medium so that
anaerobes can grow beneath its surface.
(2)
Oxygen also may be eliminated from an anaerobic system by removing air with a
vacuum pump and flushing out residual O2 with nitrogen gas (figure
6.15). Often CO2 as well as nitrogen is added to the chamber
since many anaerobes require a small amount of CO2 for best growth.
(3)
One of the most popular ways of culturing small numbers of anaerobes is by use
of a Gas-Pak jar (figure 6.16). In this procedure the environment is
made anaerobic by using hydrogen and a palladium catalyst to remove O2
through the formation of water. The reducing agents in anaerobic agar also
remove oxygen, as mentioned previously.
(4)
Plastic bags or pouches make convenient containers when only a few samples are
to be incubated anaerobically. These have a catalyst and calcium carbonate to produce
an anaerobic, carbon-dioxiderich atmosphere. A special solution is added to the
pouch’s reagent compartment; petri dishes or other containers are placed in the
pouch; it then is clamped shut and placed in an incubator.
A
laboratory may make use of all these techniques since each is best suited for
different purposes.
The Influence of Pressure on Microbial Growth
Most
organisms spend their lives on land or on the surface of water, always
subjected to a pressure of 1 atmosphere (atm), and are never affected
significantly by pressure. Yet the deep sea (ocean of 1,000 m or more in depth)
is 75% of the total ocean volume. The hydrostatic pressure can reach 600 to
1,100 atm in the deep sea, while the temperature is about 2 to 3°C.
Despite
these extremes, bacteria survive and adapt. Many are barotolerant: increased
pressure does adversely affect them but not as much as it does nontolerant
bacteria. Some bacteria in the gut of deep-sea invertebrates such as amphipods
and holothurians are truly barophilic—they grow more rapidly at high
pressures.
These
gut bacteria may play an important role in nutrient recycling in the deep sea.
One barophile has been recovered from the Mariana trench near the Philippines
(depth about 10,500 m) that is actually unable to grow at pressures below about
400 to 500 atm when incubated at 2°C. Thus far, barophiles have been found
among several bacterial genera (e.g., Photobacterium, Shewanella, Colwellia).
Some members of the Archaea are thermobarophiles (e.g., Pyrococcus spp.,
Methanococcus jannaschii).
The Influence of Pressure on Microbial Growth
Our world is bombarded with electromagnetic
radiation of various types (figure 6.17). This radiation often behaves as if it
were composed of waves moving through space like waves traveling on the surface
of water. The distance between two wave crests or troughs is the wavelength. As
the wavelength of electromagnetic radiation decreases, the energy of the
radiation increases—gamma rays and X rays are much more energetic than visible
light or infrared waves. Electromagnetic radiation also acts like a stream of
energy packets called photons, each photon having a quantum of energy whose
value will depend on the wavelength of the radiation.
Sunlight is the major source of
radiation on the Earth. It includes visible light, ultraviolet (UV) radiation,
infrared rays, and radio waves. Visible light is a most conspicuous and
important aspect of our environment: all life is dependent on the ability of photosynthetic
organisms to trap the light energy of the sun. Almost 60% of the sun’s
radiation is in the infrared region rather than the visible portion of the
spectrum.
Infrared is the major source of the
Earth’s heat. At sea level, one finds very little ultraviolet radiation below
about 290 to 300 nm. UV radiation of wavelengths shorter than 287 nm is
absorbed by O2 in the Earth’s atmosphere; this process forms a layer
of ozone between 25 and 30 miles above the Earth’s surface.
The ozone layer then absorbs somewhat
longer UV rays and reforms O2. This elimination of UV radiation is
crucial because it is quite damaging to living systems. The fairly even
distribution of sunlight throughout the visible spectrum accounts for the fact
that sunlight is generally “white.”
Many forms of electromagnetic
radiation are very harmful to microorganisms. This is particularly true of ionizing
radiation, radiation of very short wavelength or high energy, which can cause
atoms to lose electrons or ionize. Two major forms of ionizing radiation are
(1) X rays, which are artificially
produced, and
(2) gamma rays, which are emitted
during radioisotope decay.
Low levels of ionizing radiation will
produce mutations and may indirectly result in death, whereas higher levels are
directly lethal.
Although microorganisms are more resistant
to ionizing radiation than larger organisms, they will still be destroyed by a
sufficiently large dose. Ionizing radiation can be used to sterilize items.
Some procaryotes (e.g., Deinococcus radiodurans) and bacterial
endospores can survive large doses of ionizing radiation.
A variety of changes in cells are due
to ionizing radiation; it breaks hydrogen bonds, oxidizes double bonds,
destroys ring structures, and polymerizes some molecules. Oxygen enhances these
destructive effects, probably through the generation of hydroxyl radicals
(OH·). Although many types of constituents can be affected, it is reasonable to
suppose that destruction of DNA is the most important cause of death.
Ultraviolet (UV) radiation, mentioned
earlier, kills all kinds of microorganisms due to its short wavelength
(approximately from 10 to 400 nm) and high energy. The most lethal UV radiation
has a wavelength of 260 nm, the wavelength most effectively absorbed by DNA.
The primary mechanism of UV damage is the formation of thymine dimers in DNA.
Two adjacent thymines in a DNA strand are covalently joined to inhibit DNA
replication and function.
This damage is repaired in several
ways. In photoreactivation, blue light is used by a photoreactivating enzyme to
split thymine dimers. A short sequence containing the thymine dimer can also be
excised and replaced. This process occurs in the absence of light and is called
dark reactivation. Damage also can be repaired by the recA protein in
recombination repair and SOS repair. When UV exposure is too heavy, the damage
is so extensive that repair is impossible.
Although very little UV radiation
below 290 to 300 nm reaches the earth’s surface, near-UV radiation between 325
and 400 nm can harm microorganisms. Exposure to near-UV radiation induces
tryptophan breakdown to toxic photoproducts. It appears that these toxic tryptophan
photoproducts plus the near-UV radiation itself produce breaks in DNA strands.
The precise mechanism is not known, although it is different from that seen
with 260 nm UV.
Visible
light is immensely beneficial because it is the source of energy for
photosynthesis. Yet even visible light, when present in sufficient intensity,
can damage or kill microbial cells.
Usually
pigments called photosensitizers and O2 are required. All
microorganisms possess pigments like chlorophyll, bacteriochlorophyll, cytochromes,
and flavins, which can absorb light energy, become excited or activated, and
act as photosensitizers.
The
excited photosensitizer (P) transfers its energy to O2 generating singlet
oxygen (1O2).
Singlet
oxygen is a very reactive, powerful oxidizing agent that will quickly destroy a
cell. It is probably the major agent employed by phagocytes to destroy engulfed
bacteria.
Many
microorganisms that are airborne or live on exposed surfaces use carotenoid
pigments for protection against photooxidation.
Carotenoids
effectively quench singlet oxygen—that is, they absorb energy from singlet
oxygen and convert it back into the unexcited ground state. Both photosynthetic
and non-photosynthetic microorganisms employ pigments in this way.
Microbial Growth in Natural Environments
The previous section surveyed the
effects on microbial growth of individual environmental factors such as water
availability, pH, and temperature. Although microbial ecology will be
introduced in more detail at a later point, we will now briefly consider the
effect of the environment as a whole on microbial growth.
Microbial Growth Limitation by Environmental Factors
The microbial environment is complex
and constantly changing. Characteristically microorganisms in a particular
location are exposed to many overlapping gradients of nutrients and various other
environmental factors. This is particularly true of microorganisms growing in
biofilms. Microorganisms will grow in “microenvironments” until an
environmental or nutritional factor limits growth. Liebig’s law of the minimum
states that the total biomass of an organism will be determined by the nutrient
present in the lowest concentration relative to the organism’s requirements.
This law applies in both the laboratory (figure 6.2) and in terrestrial and aquatic environments. An increase in a limiting essential nutrient such as phosphate will result in an increase in the microbial population until some other nutrient becomes limiting. If a specific nutrient is limiting, changes in other nutrients will have no effect.
The situation may be even more complex than this. Multiple limiting
factors can influence a population over time. Furthermore, as we have seen,
factors such as temperature, pH, light, and salinity influence microbial populations
and limit growth. Shelford’s law of tolerance states that there are limits to
environmental factors below and above which a microorganism cannot survive and
grow, regardless of the nutrient supply.
This can readily be seen for temperature
in figure 6.13. Each microorganism has a specific temperature range in which it
can grow. The same rule applies to other factors such as pH, oxygen level, and
hydrostatic pressure in the marine environment. The growth of a microorganism
depends on both the nutrient supply and its tolerance of the environmental conditions.
Biofilms.
Most microorganisms are confronted
with deficiencies that limit their activities except when excess nutrients
allow unlimited growth. Such rapid growth will quickly deplete nutrients and
possibly result in the release of toxic waste products, which will limit further
growth.
In response to low nutrient levels
(oligotrophic environments) and intense competition, many microorganisms become
more competitive in nutrient capture and exploitation of available resources.
Often the organism’s morphology will
change in order to increase its surface area and ability to absorb nutrients.
This can involve conversion of rod-shaped procaryotes to “mini” and
“ultramicro” cells or changes in the morphology of prosthecate prokaryotes (figure
6.18), in response to starvation. Nutrient deprivation induces many other
changes as discussed previously. For example, microorganisms can undergo a
step-by-step shutdown of metabolism except for housekeeping maintenance genes.
Many factors can alter nutrient levels
in oligotrophic environments. Microorganisms may sequester critical limiting
nutrients, such as iron, making them less available to competitors.
The atmosphere can contribute
essential nutrients and support microbial growth. This is seen in the
laboratory as well as natural environments. Airborne organic substances have
been found to stimulate microbial growth in dilute media, and enrichment of growth
media by airborne organic matter can allow significant populations of
microorganisms to develop. Even distilled water, which already contains traces
of organic matter, can absorb one carbon compounds from the atmosphere and grow
microorganisms.
The presence of such airborne
nutrients and microbial growth, if not detected, can affect experiments in
biochemistry and molecular biology, as well as studies of microorganisms growing
in oligotrophic environments.
Natural substances also can directly
inhibit microbial growth and reproduction in low-nutrient environments. These
agents include phenolics, tannins, ammonia, ethylene, and volatile sulfur compounds.
This may be a means by which microorganisms avoid expending limited energy
reserves until an adequate supply of nutrients becomes available. Such chemicals
are also important in plant pathology and may aid in controlling soil-borne
microbial diseases.
Counting Viable But Non-culturable Vegetative
Procaryotes
In order to study the growth of
natural procaryotic populations outside the laboratory, it is essential to
determine the number of viable microorganisms present. For most of
microbiology’s history, a viable microorganism has been defined as one that is
able to grow actively, resulting in the formation of a colony or visible turbidity
in a liquid medium.
John R. Postgate of the University of
Sussex in England was one of the first to note that microorganisms stressed by
survival in natural habitats—or in many selective laboratory media—were
particularly sensitive to secondary stresses. Such stresses can produce viable
microorganisms without the ability to grow on media normally used for their
cultivation.
To determine the growth potential of
such microorganisms, Postgate developed what is now called the Postgate
Microviability Assay, in which microorganisms are cultured in a thin agar film
under a coverslip. The ability of a cell to change its morphology, even if it
does not grow beyond the single-cell stage, indicates that the microorganism
does show “life signs.”
Since that time many workers have
developed additional sensitive microscopic and isotopic procedures to evaluate
the presence and significance of these viable but nonculturable bacteria in
both lab and field. For example, levels of fluorescent antibody and acridine
orange–stained cells often are compared with population counts obtained by the
most probable number (MPN) method and plate counts using selective and nonselective
media.
The release of radioactive-labeled
cell materials also is used to monitor stress effects on microorganisms.
Despite these advances the estimation of substrate-responsive viable cells by
Postgate’s method is still important. These studies show that even when
bacteria such as Escherichia coli, Vibrio cholerae, Klebsiella pneumoniae,
Enterobacter aerogenes, and Enterococcus faecalis have lost their
ability to grow on conventional laboratory media using standard cultural
techniques, they still might be able to play a role in infectious disease.
The situation in natural environments
with mixed populations is much more complex. Here, where often only 1 to 10% of
observable cells are able to form colonies, the microbiologist is attempting to
grow microorganisms that perhaps never have been cultured or characterized. In
the future it is possible that media or proper environmental conditions for
their growth will be developed.
At present, molecular techniques
involving PCR amplification and small subunit ribosomal RNA analysis are
increasingly used to analyze the diversity of uncultured microbial populations.
Quorum Sensing and Microbial Populations
For decades microbiologists tended to
think of bacterial populations as collections of individuals growing and
behaving independently.
More recently it has become clear that
many bacteria can communicate with one another and behave cooperatively. A major
way in which this cooperation is accomplished is by a process known as quorum
sensing or autoinduction. This is a phenomenon in which bacteria monitor their
own population density through sensing the levels of signal molecules,
sometimes called autoinducers because they can stimulate the cell that releases
them.
The concentration of these signal
molecules increases along with the bacterial population until it rises to a
specific threshold and signals the bacteria that the population density has reached
a critical level or quorum. The bacteria then begin expressing sets of
quorum-dependent genes. Quorum sensing has been found among both gram-negative
and gram-positive bacteria.
Quorum sensing makes good practical
sense. Take the production and release of extracellular enzymes as an example.
If such enzymes were released by only a few bacteria, they would diffuse away
and be rendered ineffective because of dilution.
With control by quorum sensing, the
bacteria reach a high population density before they release enzymes, and as a
consequence enzyme levels are concentrated enough to have significant effects.
This is an advantage within a host’s
body as well as in the soil or an aquatic habitat. If a pathogen can reach high
levels at a particular site before producing virulence factors and escaping into
surrounding host tissues, it has a much better chance of counteracting host
defenses and successfully spreading throughout the host’s body. This explains
another pattern in quorum sensing. It seems to be very important in many
bacteria that establish symbiotic or parasitic relationships with hosts.
Quorum sensing was first discovered in gram-negative bacteria and is best understood in these microorganisms. The most common signals in gram-negative bacteria are acyl homoserine lactones (HSLs). These are small molecules composed of a 4- to 14-carbon acyl chain attached by an amide bond to homoserine lactone (figure 6.19a). The acyl chain may have a keto group or hydroxyl group on its third carbon.
Acyl HSLs
diffuse into the target cell (figure 6.19b). Once they reach a
sufficiently high level, acyl HSLs bind to special receptor proteins and
trigger a conformational change. Usually the activated complexes act as inducers—that
is, they bind to target sites on the DNA and stimulate transcription of
quorum-sensitive genes. The gene needed to synthesize acyl HSL is also produced
frequently, thus amplifying the effect by the production and release of more
autoinducer molecules.
Many different processes are sensitive
to acyl HSL signals and quorum sensing in gram-negative bacteria. Some
well-studied examples are (1) bioluminescence production by Vibrio fischeri,
(2) Pseudomonas aeruginosa synthesis and release of virulence factors,
(3) conjugal transfer of genetic material by Agrobacterium tumefaciens, and
(4) antibiotic production by Erwinia carotovora and Pseudomonas
aureofaciens.
Gram-positive bacteria also regulate
activities by quorum sensing, often using an oligopeptide signal. Good examples
are mating in Enterococcus faecalis, competence induction in Streptococcus
pneumoniae, stimulation of sporulation by Bacillus subtilis, and
production of many toxins and other virulence factors by Staphylococcus
aureus. Quorum sensing even stimulates the development of aerial mycelia
and the production of streptomycin by Streptomyces griseus. In this
case, the signal seems to be γ-butyrolactone rather than an oligopeptide.
An interesting and important function
of quorum sensing is to promote the formation of mature biofilms by the
pathogen Pseudomonas aeruginosa, and it may play a role in cystic
fibrosis.
Biofilm formation makes sense for the
pathogen because biofilms protect against antibiotics and detergents. Quorum
sensing should be very effective within biofilms because there will be less
dilution and acyl HSL levels will increase rapidly. Under such circumstances, two
different bacteria might stimulate each other by releasing similar signals;
this appears to be the case in biofilms containing the pathogens P.
aeruginosa and Burkholderia cepacia.
Quorum sensing is an example of what
might be called multicellular behavior in that many individual cells
communicate and coordinate their activities to act as a unit. Other examples of
such complex behavior is pattern formation in colonies and fruiting body
formation in the myxobacteria.
















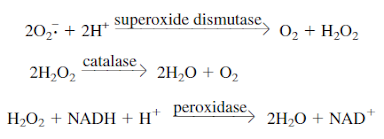






No comments:
Post a Comment