In Procaryotic chapter, considerable attention is devoted to prokaryotic cell structure and function because bacteria are immensely important in microbiology and have occupied a large portion of microbiologists’ attention in the past. Nevertheless, eucaryotic algae, fungi, and protozoa also are microorganisms and have been extensively studied.
These
organisms often are extraordinarily complex, interesting in their own right,
and prominent members of the ecosystem (figure 4.1). In addition, fungi
(and to some extent, algae) are exceptionally useful in industrial microbiology.
Many fungi and protozoa are also major human pathogens; one only need think of
either malaria or African sleeping sickness to appreciate
the significance of eucaryotes in pathogenic microbiology. So, although this
text emphasizes bacteria, eucaryotic microorganisms are discussed at many
points.
This
chapter focuses on eucaryotic cell structure and its relationship to cell
function. Because many valuable studies on eukaryotic cell ultrastructure have
used organisms other than microorganisms, some work on nonmicrobial cells is
presented. At the end of the chapter, procaryotic and eucaryotic cells are
compared in some depth.
An Overview of Eucaryotic Cell Structure
The
most obvious difference between eucaryotic and prokaryotic cells is in their
use of membranes. Eucaryotic cells have membrane delimited nuclei, and
membranes also play a prominent part in the structure of many other organelles
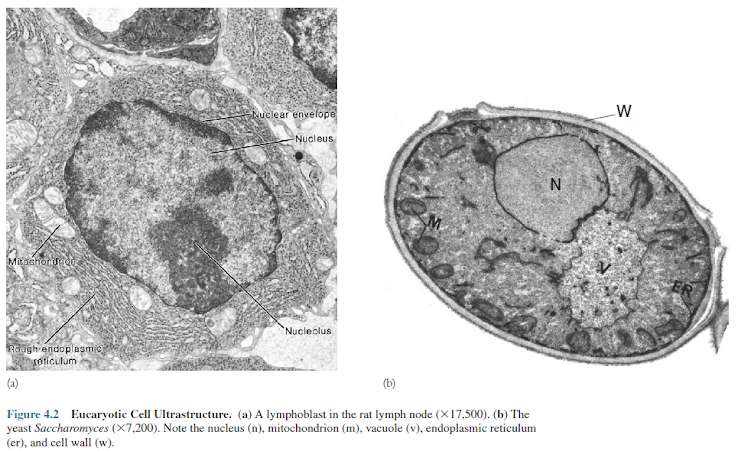
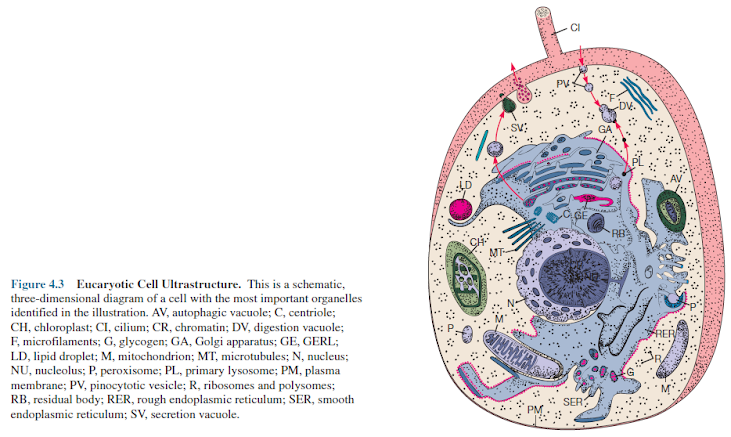
(figures 4.2 and 4.3).
Organelles are intracellular structures that perform specific functions in cells analogous to the functions of organs in the body. The name organelle (little organ) was coined because biologists saw a parallel between the relationship of organelles to a cell and that of organs to the whole body. It is not satisfactory to define organelles as membrane- bound structures because this would exclude such components as ribosomes and bacterial flagella. A comparison of figures 4.2 and 4.3 shows how much more structurally complex the eucaryotic cell is.
This complexity is due chiefly to the use of internal membranes for several purposes. The partitioning of the eucaryotic cell interior by membranes makes possible the placement of different biochemical and physiological functions in separate compartments so that they can more easily take place simultaneously under independent control and proper coordination.
Large
membrane surfaces make possible greater respiratory and photosynthetic activity
because these processes are located exclusively in membranes. The
intracytoplasmic membrane complex also serves as a transport system to move
materials between different cell locations. Thus abundant membrane systems probably
are necessary in eucaryotic cells because of their large volume and the need
for adequate regulation, metabolic activity, and transport.
Figures
4.2, 4.3, and 4.26b provide generalized views of eukaryotic cell
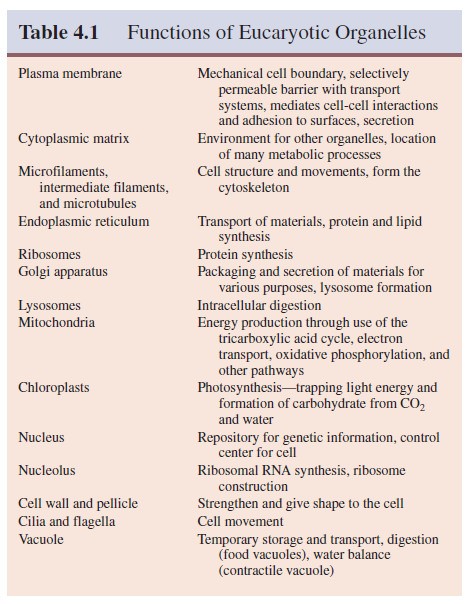
structure and illustrate most of the organelles to be discussed. Table 4.1 briefly
summarizes the functions of the major eucaryotic organelles. Those organelles
lying inside the plasma membrane are first described, and then components
outside the membrane are discussed.
The Cytoplasmic Matrix, Microfilaments, Intermediate Filaments,
and Microtubules
When
a eucaryotic cell is examined at low power with the electron microscope, its
larger organelles are seen to lie in an apparently featureless, homogeneous
substance called the cytoplasmic matrix. The matrix, although
superficially uninteresting, is actually one of the most important and complex
parts of the cell. It is the “environment” of the organelles and the location
of many important biochemical processes. Several physical changes seen in cells—viscosity
changes, cytoplasmic streaming, and others—also are due to matrix activity.
Water
constitutes about 70 to 85% by weight of a eukaryotic cell. Thus a large part
of the cytoplasmic matrix is water. Cellular water can exist in two different
forms. Some of it is bulk or free water; this is normal, osmotically active
water. Osmosis, water activity, and growth Water also can exist as bound water
or water of hydration.
This
water is bound to the surface of proteins and other macromolecules and is
osmotically inactive and more ordered than bulk water. There is some evidence
that bound water is the site of many metabolic processes. The protein content
of cells is so high that the cytoplasmic matrix often may be semi-crystalline.
Usually matrix pH is around neutrality, about pH 6.8 to 7.1, but can vary widely.
For example, protozoan digestive vacuoles may reach pHs as low as 3 to 4.
Probably
all eucaryotic cells have microfilaments, minute protein filaments, 4 to
7 nm in diameter, which may be either scattered within the cytoplasmic matrix
or organized into networks and parallel arrays. Microfilaments are involved in
cell motion and shape changes. Some examples of cellular movements associated with
microfilament activity are the motion of pigment granules, amoeboid movement,
and protoplasmic streaming in slime molds.
The
participation of microfilaments in cell movement is suggested by electron
microscopic studies showing that they frequently are found at locations
appropriate for such a role. For example, they are concentrated at the interface
between stationary and flowing cytoplasm in plant cells and slime molds.
Experiments using the drug cytochalasin B have provided additional evidence.
Cytochalasin B disrupts microfilament structure and often simultaneously
inhibits cell movements. However, because the drug has additional effects in
cells, a direct cause-and-effect interpretation of these experiments is
sometimes difficult.
Microfilament
protein has been isolated and analyzed chemically. It is an actin, very similar
to the actin contractile protein of muscle tissue. This is further indirect
evidence for microfilament involvement in cell movement.
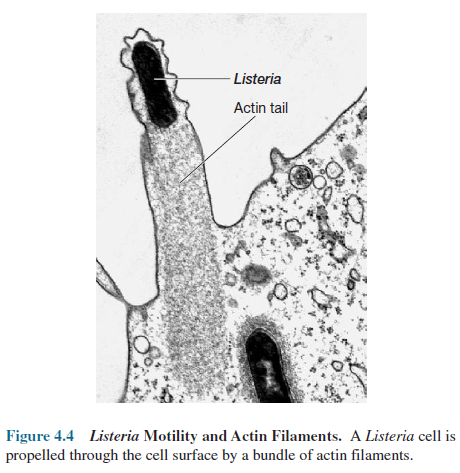
Some
pathogens such as Listeria monocytogenes make use of eucaryotic actin to
move rapidly through the host cell. The ActA protein released by Listeria causes
the polymerization of actin filaments at the end of the bacterium. A tail of
actin is formed and trapped in the host cytoskeleton. Its continued elongation pushes
the bacterium along at rates up to 11 µm/minute.
The
bacterium can even be propelled through the cell surface and into neighboring
cells (figure 4.4). A second type of small filamentous organelle in the
cytoplasmic matrix is shaped like a thin cylinder about 25 nm in diameter.
Because
of its tubular nature this organelle is called a microtubule. Microtubules
are complex structures constructed of two slightly different spherical
protein subunits named tubulins, each of which is approximately 4 to 5 nm in
diameter. These subunits are assembled in a helical arrangement to form a
cylinder with an average of 13 subunits in one turn or circumference (figure
4.5).
Microtubules
serve at least three purposes:
(1) they help maintain cell shape,
(2) are involved with microfilaments in cell movements, and
(3) participate in intracellular transport processes.
Evidence for a structural role comes from their intracellular distribution and studies on the effects of the drug colchicine. Long, thin cell structures requiring support such as the axopodia (long, slender, rigid pseudopodia) of protozoa contain microtubules (figure 4.6).
When migrating embryonic nerve and
heart cells are exposed to colchicine, they simultaneously lose their
microtubules and their characteristic shapes. The shapeless cells seem to
wander aimlessly as if incapable of directed movement without their normal
form. Their microfilaments are still intact, but due to the disruption of their
microtubules by colchicine, they no longer behave normally.
Microtubules
also are present in structures that participate in cell or organelle
movements—the mitotic spindle, cilia, and flagella.
For
example, the mitotic spindle is constructed of microtubules; when a dividing
cell is treated with colchicine, the spindle is disrupted and chromosome
separation blocked. Microtubules also are essential to the movement of
eucaryotic cilia and flagella.
Other
kinds of filamentous components also are present in the matrix, the most
important of which are the intermediate filaments (about 8 to 10 nm in
diameter). The microfilaments, microtubules, and intermediate filaments are
major components of a vast, intricate network of interconnected filaments
called the cytoskeleton (figure 4.7). As mentioned previously,
the cytoskeleton plays a role in both cell shape and movement. Procaryotes lack
a true, organized cytoskeleton and may not possess actinlike proteins.
The
Endoplasmic Reticulum
Besides the cytoskeleton, the cytoplasmic matrix is permeated with an irregular network of branching and fusing membranous tubules, around 40 to 70 nm in diameter, and many flattened sacs called cisternae (s., cisterna). This network of tubules and cisternae is the endoplasmic reticulum (ER) (figure 4.2a and figure 4.8). The nature of the ER varies with the functional and physiological status of the cell.
In cells synthesizing a great deal of protein for purposes such as
secretion, a large part of the ER is studded on its outer surface with
ribosomes and is called rough or granular endoplasmic reticulum (RER or GER).
Other cells, such as those producing large quantities of lipids, have ER that lacks
ribosomes. This is smooth or agranular ER (SER orAER).
The
endoplasmic reticulum has many important functions. It transports proteins,
lipids, and probably other materials through the cell. Lipids and proteins are
synthesized by ER-associated enzymes and ribosomes. Polypeptide chains
synthesized on RERbound ribosomes may be inserted either into the ER membrane
or into its lumen for transport elsewhere. The ER is also a major site of cell
membrane synthesis.
New endoplasmic
reticulum is produced through expansion of the old. Many biologists think the
RER synthesizes new ER proteins and lipids. “Older” RER then loses its
connected ribosomes and is modified to become SER. Not everyone agrees with this
interpretation, and other mechanisms of growth of ER are possible.
The Golgi
Apparatus
The Golgi apparatus is a membranous organelle composed of flattened, saclike cisternae stacked on each other (figure 4.9). These membranes, like the smooth ER, lack bound ribosomes. There are usually around 4 to 8 cisternae or sacs in a stack, although there may be many more.
Each sac is 15 to 20 nm thick and
separated from other cisternae by 20 to 30 nm. A complex network of tubules and
vesicles (20 to 100 nm in diameter) is located at the edges of the cisternae.
The stack of cisternae has a definite polarity because there are two ends or
faces that are quite different from one another.
The
sacs on the cis or forming face often are associated with the ER and differ
from the sacs on the trans or maturing face in thickness, enzyme content, and
degree of vesicle formation. It appears that material is transported from cis
to trans cisternae by vesicles that bud off the cisternal edges and move to the
next sac.
The
Golgi apparatus is present in most eucaryotic cells, but many fungi and ciliate
protozoa may lack a well-formed structure.
Sometimes
it consists of a single stack of cisternae; however, many cells may contain up
to 20, and sometimes more, separate stacks. These stacks of cisternae, often
called dictyosomes, can be clustered in one region or scattered about the cell.
The Golgi apparatus packages materials and prepares them for secretion, the
exact nature of its role varying with the organism.
The
surface scales of some flagellated algae and radiolarian protozoa appear to be
constructed within the Golgi apparatus and then transported to the surface in
vesicles. It often participates in the development of cell membranes and in the
packaging of cell products. The growth of some fungal hyphae occurs when Golgi
vesicles contribute their contents to the wall at the hyphal tip.
In
all these processes, materials move from the ER to the Golgi apparatus. Most
often vesicles bud off the ER, travel to the Golgi apparatus, and fuse with the
cis cisternae. Thus the Golgi apparatus is closely related to the ER in both a
structural and a functional sense. Most proteins entering the Golgi apparatus
from the ER are glycoproteins containing short carbohydrate chains.
The
Golgi apparatus frequently modifies proteins destined for different fates by
adding specific groups and then sends the proteins on their way to the proper
location (e.g., lysosomal proteins have phosphates added to their mannose
sugars).
Lysosomes and
Endocytosis
A very important function of the Golgi apparatus and endoplasmic reticulum is the synthesis of another organelle, the lysosome. This organelle (or a structure very much like it) is found in a variety of microorganisms—protozoa, some algae, and fungi—as well as in plants and animals. Lysosomes are roughly spherical and enclosed in a single membrane; they average about 500 nm in diameter, but range from 50 nm to several µm in size. They are involved in intracellular digestion and contain the enzymes needed to digest all types of macromolecules. These enzymes, called hydrolases, catalyze the hydrolysis of molecules and function best under slightly acid conditions (usually around pH 3.5 to 5.0). Lysosomes maintain an acidic environment by pumping protons into their interior.
Digestive
enzymes are manufactured by the RER and packaged to form lysosomes by the Golgi
apparatus. A segment of smooth ER near the Golgi apparatus also may bud off
lysosomes.
Lysosomes
are particularly important in those cells that obtain nutrients through
endocytosis. In this process a cell takes up solutes or particles by enclosing
them in vacuoles and vesicles pinched off from its plasma membrane. Vacuoles
and vesicles are membrane delimited cavities that contain fluid, and often
solid material. Larger cavities will be called vacuoles, and smaller cavities,
vesicles. There are two major forms of endocytosis: phagocytosis and
pinocytosis.
During
phagocytosis large particles and even other microorganisms are enclosed in a
phagocytic vacuole or phagosome and engulfed (figure 4.10a). In
pinocytosis small amounts of the surrounding liquid with its solute molecules
are pinched off as tiny pinocytotic vesicles (also called pinocytic vesicles)
or pinosomes. Often phagosomes and pinosomes are collectively called endosomes
because they are formed by endocytosis. The type of pinocytosis, receptor mediated
endocytosis, that produces coated vesicles is important in the entry of animal
viruses into host cells.
Material
in endosomes is digested with the aid of lysosomes. Newly formed lysosomes, or primary
lysosomes, fuse with phagocytic vacuoles to yield secondary lysosomes, lysosomes
with material being digested (figure 4.10). These phagocytic vacuoles or
secondary lysosomes often are called food vacuoles. Digested nutrients then
leave the secondary lysosome and enter the cytoplasm. When the lysosome has
accumulated large quantities of indigestible material, it is known as a residual
body.
Lysosomes
join with phagosomes for defensive purposes as well as to acquire nutrients.
Invading bacteria, ingested by a phagocytic cell, usually are destroyed when
lysosomes fuse with the phagosome. This is commonly seen in leukocytes (white
blood cells) of vertebrates.
Cells
can selectively digest portions of their own cytoplasm in a type of secondary
lysosome called an autophagic vacuole (figure 4.10a). It is
thought that these arise by lysosomal engulfment of a piece of cytoplasm (figure
4.11), or when the ER pinches off cytoplasm to form a vesicle that
subsequently fuses with lysosomes. Autophagy probably plays a role in the
normal turnover or recycling of cell constituents. A cell also can survive a
period of starvation by selectively digesting portions of itself to remain
alive. Following cell death, lysosomes aid in digestion and removal of cell
debris.
A
most remarkable thing about lysosomes is that they accomplish all these tasks
without releasing their digestive enzymes into the cytoplasmic matrix, a
catastrophe that would destroy the cell. The lysosomal membrane retains
digestive enzymes and other macromolecules while allowing small digestion products
to leave.
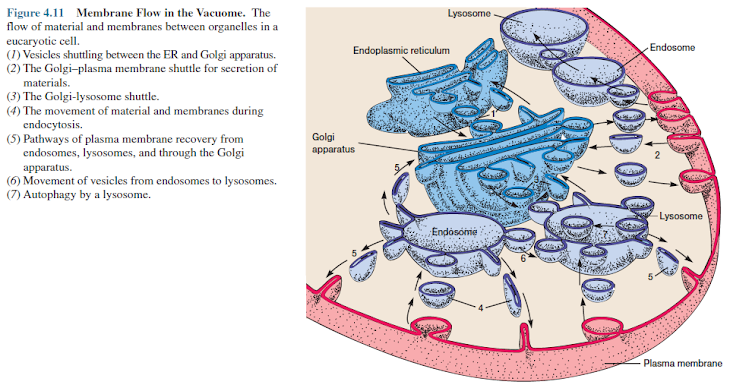
The
intricate complex of membranous organelles composed of the Golgi apparatus,
lysosomes, endosomes, and associated structures seems to operate as a coordinated
whole whose main function is the import and export of materials (figure 4.11).
Christian de Duve (Nobel Prize, 1974) has suggested that this complex be called
the vacuome in recognition of its functional unity. The ER manufactures
secretory proteins and membrane, and contributes these to the Golgi apparatus.
The
Golgi apparatus then forms secretory vesicles that fuse with the plasma
membrane and release material to the outside. It also produces lysosomes that
fuse with endosomes to digest material acquired through phagocytosis and
pinocytosis.
Membrane
movement in the region of the vacuome lying between the Golgi apparatus and the
plasma membrane is two-way. Empty vesicles often are recycled and returned to
the Golgi apparatus and plasma membrane rather than being destroyed.
These
exchanges in the vacuome occur without membrane rupture so that vesicle
contents never escape directly into the cytoplasmic matrix.
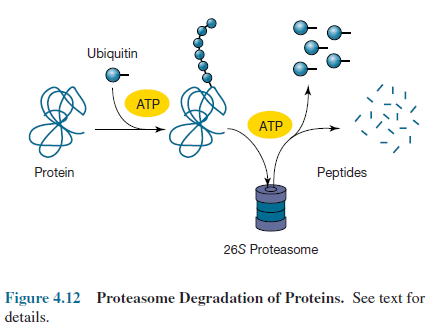
More
recently a nonlysosomal protein degradation system has been discovered in
eucaryotic cells, a few bacteria, and many archaea. The majority of eucaryotic
proteins may be degraded by this system. In eucaryotes, proteins are targeted
for destruction by the attachment of several small ubiquitin polypeptides (figure
4.12). The marked protein then enters a huge cylindrical complex called a
26S proteasome, where it is degraded to peptides in an ATP-dependent
process and the ubiquitins are released. The peptides may be hydrolyzed to
amino acids. In this case the system is being used to recycle proteins. The
proteasome also is involved in producing peptides for antigen presentation
during many immunological responses.
Eucaryotic Ribosomes
The
eucaryotic ribosome can either be associated with the endoplasmic reticulum or
be free in the cytoplasmic matrix and is larger than the bacterial 70S
ribosome. It is a dimer of a 60S and a 40S subunit, about 22 nm in diameter,
and has a sedimentation coefficient of 80S and a molecular weight of 4 million.
When bound to the endoplasmic reticulum to form rough ER, it is attached through
its 60S subunit.
Both
free and RER-bound ribosomes synthesize proteins. As mentioned earlier,
proteins made on the ribosomes of the RER either enter its lumen for transport,
and often for secretion, or are inserted into the ER membrane as integral
membrane proteins.
Free
ribosomes are the sites of synthesis for nonsecretory and nonmembrane proteins.
Some proteins synthesized by free ribosomes are inserted into organelles such
as the nucleus, mitochondrion, and chloroplast. Molecular chaperones aid the
proper folding of proteins after synthesis. They also assist the transport of
proteins into eucaryotic organelles such as mitochondria. Several ribosomes usually
attach to a single messenger RNA and simultaneously translate its message into
protein. These complexes of messenger RNA and ribosomes are called polyribosomes
or polysomes. Ribosomal participation in protein synthesis is dealt with
later.
Mitochondria
Found
in most eucaryotic cells, mitochondria (s., mitochondrion) frequently
are called the “powerhouses” of the cell. Tricarboxylic acid cycle activity and
the generation of ATP by electron transport and oxidative phosphorylation take
place here. In the transmission electron microscope, mitochondria usually are cylindrical
structures and measure approximately 0.3 to 1.0 µm by 5 to 10 µm. (In other
words, they are about the same size as bacterial cells.) Although cells can
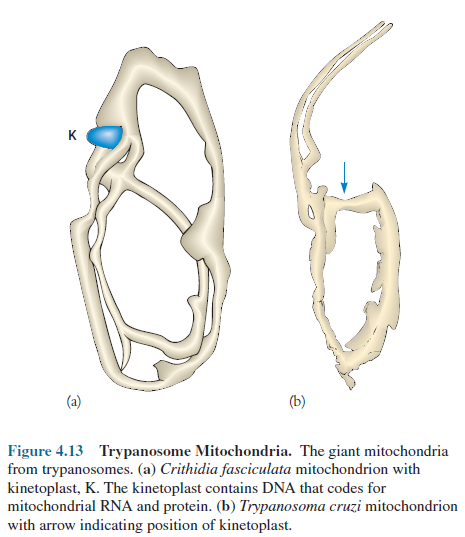
possess as many as 1,000 or more mitochondria, at least a few cells (some
yeasts, unicellular algae, and trypanosome protozoa) have a single giant
tubular mitochondrion twisted into a continuous network permeating the cytoplasm
(figure 4.13).
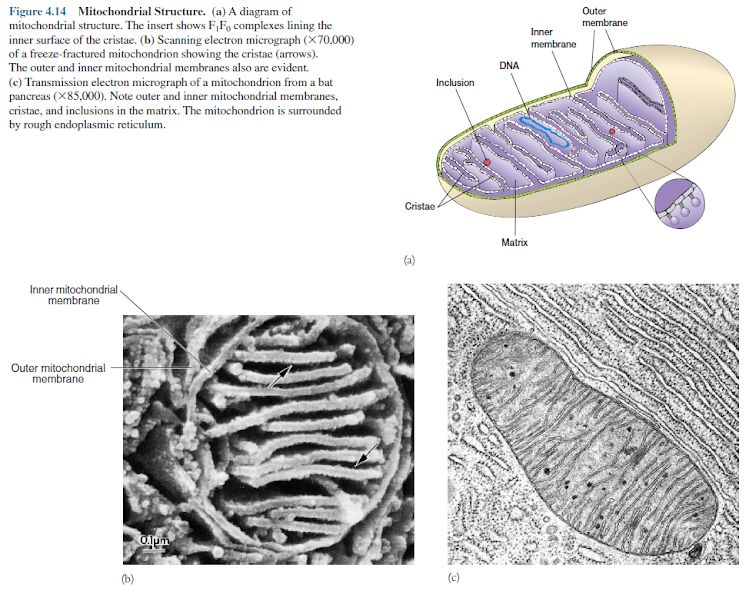
The
mitochondrion is bounded by two membranes, an outer mitochondrial membrane
separated from an inner mitochondrial membrane by a 6 to 8 nm intermembrane
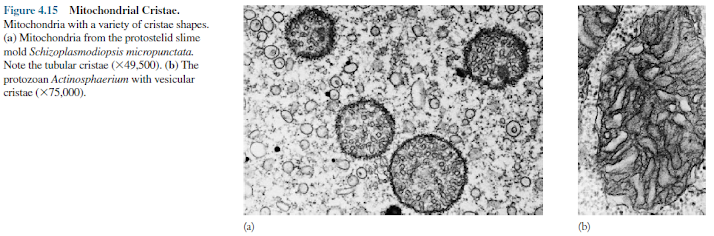
space (figure 4.14). Special infoldings of the inner membrane, called cristae
(s., crista), greatly increase its surface area. Their shape differs
in mitochondria from various species. Fungi have plate-like (laminar) cristae, whereas
euglenoid flagellates may have cristae shaped like disks.
Tubular
cristae are found in a variety of eucaryotes; however, amoebae can possess
mitochondria with cristae in the shape of vesicles (figure 4.15). The
inner membrane encloses the mitochondrial matrix, a dense matrix containing
ribosomes, DNA, and often large calcium phosphate granules. Mitochondrial
ribosomes are smaller than cytoplasmic ribosomes and resemble those of bacteria
in several ways, including their size and subunit composition.
Mitochondrial
DNA is a closed circle like bacterial DNA. Each mitochondrial compartment is
different from the others in chemical and enzymatic composition. The outer and
inner mitochondrial membranes, for example, possess different lipids. Enzymes and
electron carriers involved in electron transport and oxidative phosphorylation
(the formation of ATP as a consequence of electron transport) are located only
in the inner membrane. The enzymes of the tricarboxylic acid cycle and the β-oxidation
pathway for fatty acids are located in the matrix.
The
inner membrane of the mitochondrion has another distinctive structural feature
related to its function. Many small spheres, about 8.5 nm diameter, are
attached by stalks to its inner surface. The spheres are called F1 particles
and synthesize ATP during cellular respiration.
The
mitochondrion uses its DNA and ribosomes to synthesize some of its own
proteins. In fact, mutations in mitochondrial DNA often lead to serious
diseases in humans. Most mitochondrial proteins, however, are manufactured
under the direction of the nucleus. Mitochondria reproduce by binary fission.
Chloroplasts show similar partial independence and reproduction by binary fission.
Because both organelles resemble bacteria to some extent, it has been suggested
that these organelles arose from symbiotic associations between bacteria and
larger cells (Box 4.1).
Chloroplasts
Plastids
are cytoplasmic organelles of algae and higher plants that often possess pigments
such as chlorophylls and carotenoids, and are the sites of synthesis and
storage of food reserves.
The
most important type of plastid is the chloroplast. Chloroplasts contain
chlorophyll and use light energy to convert CO2 and water to
carbohydrates and O2. That is, they are the site of photosynthesis.
Although
chloroplasts are quite variable in size and shape, they share many structural
features. Most often they are oval with dimensions of 2 to 4 µm by 5 to 10 µm,
but some algae possess one huge chloroplast that fills much of the cell. Like
mitochondria,
chloroplasts
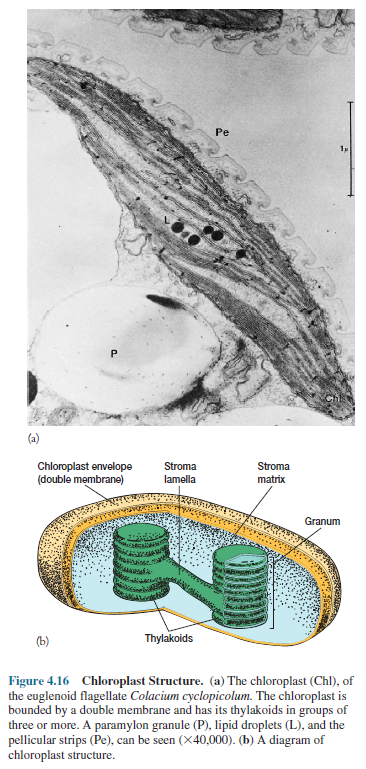
are encompassed by two membranes (figure 4.16). A matrix, the stroma, lies
within the inner membrane. It contains DNA, ribosomes, lipid droplets, starch
granules, and a complex internal membrane system whose most prominent
components are flattened, membrane-delimited sacs, the thylakoids. Clusters of two
or more thylakoids are dispersed within the stroma of most algal chloroplasts
(figures 4.16 and 4.25b). In some groups of algae, several disk-like
thylakoids are stacked on each other like coins to form grana (s., granum).
Photosynthetic
reactions are separated structurally in the chloroplast just as electron
transport and the tricarboxylic acid cycle are in the mitochondrion. The
formation of carbohydrate from CO2 and water, the dark reaction, takes place in
the stroma.
The
trapping of light energy to generate ATP, NADPH, and O2, the light reaction,
is located in the thylakoid membranes, where chlorophyll and electron transport
components are also found.
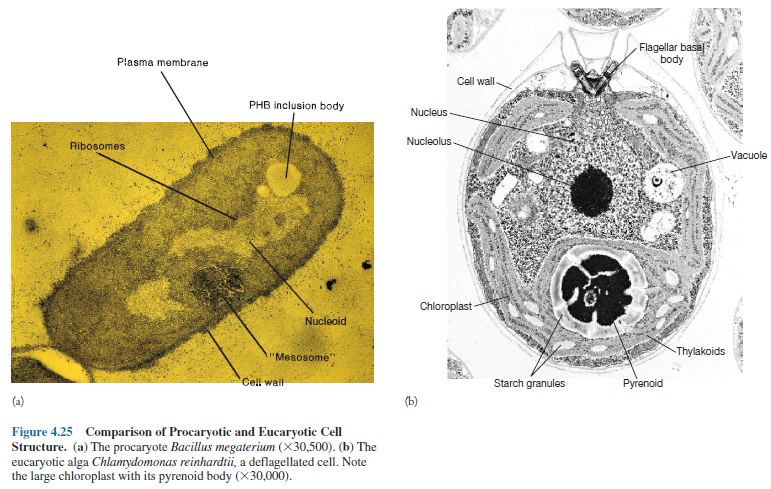
The
chloroplasts of many algae contain a pyrenoid (figure 4.25b), a dense
region of protein surrounded by starch or another polysaccharide. Pyrenoids
participate in polysaccharide synthesis.
The Nucleus
and Cell Division
The
cell nucleus is by far the most visually prominent organelle. It was discovered
early in the study of cell structure and was shown by Robert Brown in 1831 to
be a constant feature of eukaryotic cells. The nucleus is the repository for
the cell’s genetic information and is its control center.
Nuclear
Structure
Nuclei
are membrane-delimited spherical bodies about 5 to 7 µm in diameter (figures
4.2 and 4.25b). Dense fibrous material called chromatin can be seen
within the nucleoplasm of the nucleus of a stained cell. This is the
DNA-containing part of the nucleus. In nondividing
cells, chromatin exists in a dispersed condition, but condenses during mitosis
to become visible as chromosomes.
Some
nuclear chromatin, the euchromatin, is loosely organized and contains those
genes that are expressing themselves actively.
Heterochromatin
is coiled more tightly, appears darker in the electron microscope, and is not
genetically active most of the time. Organization of DNA in eucaryotic nuclei The
nucleus is bounded by the nuclear envelope (figures 4.2 and 4.25b), a
complex structure consisting of inner and outer membranes separated by a 15 to
75 nm perinuclear space. The envelope is continuous with the ER at several
points and its outer membrane is covered with ribosomes. A network of intermediate
filaments, called the nuclear lamina, lies against the inner surface of the
envelope and supports it. Chromatin usually is associated with the inner
membrane.
Many
nuclear pores penetrate the envelope (figure 4.17), each pore formed by a
fusion of the outer and inner membranes.
Pores
are about 70 nm in diameter and collectively occupy about 10 to 25% of the
nuclear surface. A complex ring-like arrangement of granular and fibrous
material called the annulus is located at the edge of each pore.
The
nuclear pores serve as a transport route between the nucleus and surrounding
cytoplasm. Particles have been observed moving into the nucleus through the
pores. Although the function of the annulus is not understood, it may either
regulate or aid the movement of material through the pores. Substances also
move directly through the nuclear envelope by unknown mechanisms.
The Nucleolus
Often
the most noticeable structure within the nucleus is the nucleolus (figures 4.2
and 4.25b). A nucleus may contain from one to many nucleoli. Although
the nucleolus is not membrane-enclosed, it is a complex organelle with separate
granular and fibrillar regions.
It is
present in nondividing cells, but frequently disappears during mitosis. After
mitosis the nucleolus reforms around the nucleolar organizer, a particular part
of a specific chromosome.
The
nucleolus plays a major role in ribosome synthesis. The nucleolar organizer DNA
directs the production of ribosomal RNA (rRNA). This RNA is synthesized in a
single long piece that then is cut to form the final rRNA molecules. The
processed rRNAs next combine with ribosomal proteins (which have been synthesized
in the cytoplasmic matrix) to form partially completed ribosomal subunits. The
granules seen in the nucleolus are probably these subunits. Immature ribosomal
subunits then leave the nucleus, presumably by way of the nuclear envelope
pores and mature in the cytoplasm.
Mitosis and
Meiosis
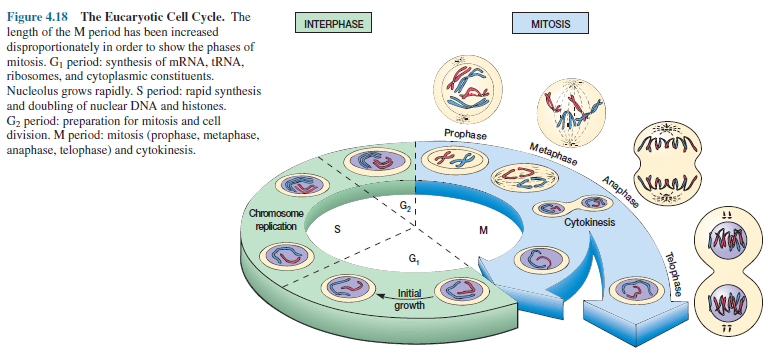
When a eucaryotic microorganism reproduces, its genetic material must be duplicated and then separated so that each new nucleus possesses a complete set of chromosomes. This process of nuclear division and chromosome distribution in eucaryotic cells is called mitosis. Mitosis actually occupies only a small portion of a microorganism’s life as can be seen by examining the cell cycle (figure 4.18).
The cell cycle is the total sequence of events in the growth-division
cycle between the end of one division and the end of the next. Cell growth
takes place in the interphase, that portion of the cycle between periods of
mitosis. Interphase is composed of three parts. The G1 period (gap 1 period) is
a time of active synthesis of RNA, ribosomes, and other cytoplasmic constituents
accompanied by considerable cell growth.
This
is followed by the S period (synthesis period) in which DNA is replicated and
doubles in quantity. Finally, there is a second gap, the G2 period, when the
cell prepares for mitosis, the M period, by activities such as the synthesis of
special division proteins. The total length of the cycle differs considerably
between microorganisms, usually due to variations in the length of G1.
Mitotic
events are summarized in figure 4.18. During mitosis, the genetic material
duplicated during the S period is distributed equally to the two new nuclei so
that each has a full set of genes.
There are four phases in mitosis. In prophase, the chromosomes— each with two chromatids—become visible and move toward the equator of the cell. The mitotic spindle forms, the nucleolus disappears, and the nuclear envelope begins to dissolve. The chromosomes are arranged in the center of the spindle during metaphase and the nuclear envelope has disappeared.
During anaphase the chromatids
in each chromosome separate and move toward the opposite poles of the spindle.
Finally during telophase the chromatids become less visible, the nucleolus
reappears, and a nuclear envelope reassembles around each set of chromatids to
form two new nuclei.
Mitosis
in eucaryotic microorganisms can differ from that pictured in figure 4.18. For
example, the nuclear envelope does not disappear in many fungi and some
protozoa and algae (figure 4.19).
Frequently
cytokinesis, the division of the parental cell’s cytoplasm to form new cells,
begins during anaphase and finishes by the end of telophase. However, mitosis
can take place without cytokinesis to generate multinucleate or coenocytic
cells.
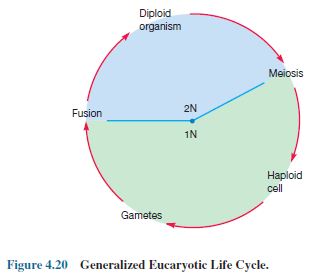
In
mitosis the original number of chromosomes is the same after division and a
diploid organism will remain diploid or 2N (i.e., it still has two copies of
each chromosome). Frequently a microorganism reduces its chromosome number by
half, from the diploid state to the haploid or 1N (a single copy of each
chromosome).
Haploid
cells may immediately act as gametes and fuse to reform diploid organisms or
may form gametes only after a considerable delay (figure 4.20). The process by
which the number of chromosomes is reduced in half with each daughter cell
receiving one complete set of chromosomes is called meiosis. Life cycles can be
quite complex in eucaryotic microorganisms; a classic example is the life cycle
of Plasmodium, the cause of malaria.
Meiosis
is quite complex and involves two stages. The first stage differs markedly from
mitosis. During prophase, homologous chromosomes come together and lie
side-by-side, a process known as synapsis. Then the double-stranded chromosomes
from each homologous pair move to opposite poles in anaphase. In contrast, during
mitotic anaphase the two strands of each chromosome separate and move to opposite
poles. Consequently the number of chromosomes is halved in meiosis but not in
mitosis. The second stage of meiosis is similar to mitosis in terms of
mechanics, and single-stranded chromosomes are separated. After completion of meiosis
I and meiosis II, the original diploid cell has been transformed into four
haploid cells.
External Cell
Coverings
Eucaryotic
microorganisms differ greatly from procaryotes in the supporting or protective
structures they have external to the plasma membrane. In contrast with most bacteria,
many eucaryotes lack an external cell wall. The amoeba is an excellent example.
Eucaryotic cell membranes, unlike most procaryotic membranes, contain sterols
such as cholesterol in their lipid bilayers, and this may make them mechanically
stronger, thus reducing the need for external support. (However, as mentioned
on page 47, many prokaryotic membranes are strengthened by hopanoids.)
Of
course many eucaryotes do have a rigid external cell wall. Algal cell walls
usually have a layered appearance and contain large quantities of
polysaccharides such as cellulose and pectin. In addition, inorganic substances
like silica (in diatoms) or calcium carbonate (some red algae) may be present.
Fungal cell walls normally are rigid.
Their
exact composition varies with the organism; but usually, cellulose, chitin, or
glucan (a glucose polymer different from cellulose) are present. Despite their
nature the rigid materials in eucaryotic walls are chemically simpler than
procaryotic peptidoglycan.
Many protozoa and some algae have a different external structure, the pellicle (figure 4.16a). This is a relatively rigid layer of components just beneath the plasma membrane (sometimes the plasma membrane is also considered part of the pellicle). The pellicle may be fairly simple in structure.
For example, Euglena has a
series of overlapping strips with a ridge at the edge of each strip fitting
into a groove on the adjacent one. In contrast, ciliate protozoan pellicles are
exceptionally complex with two membranes and a variety of associated
structures. Although pellicles are not as strong and rigid as cell walls, they
do give their possessors a characteristic shape.
Cilia and
Flagella
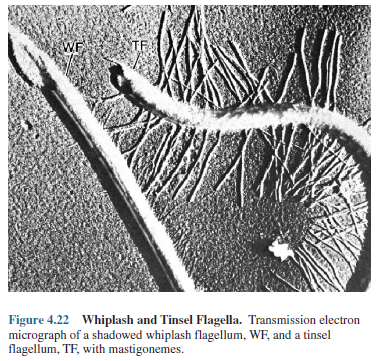
Cilia (s., cilium) and flagella (s., flagellum) are the most prominent organelles associated with motility. Although both are whip-like and beat to move the microorganism along, they differ from one another in two ways. First, cilia are typically only 5 to20 µm in length, whereas flagella are 100 to 200 µm long. Second, their patterns of movement are usually distinctive (figure 4.21).
Flagella
move in an undulating fashion and generate planar or helical waves originating
at either the base or the tip. If the wave moves from base to tip, the cell is
pushed along; a beat traveling from the tip toward the base pulls the cell through
the water. Sometimes the flagellum will have lateral hairs called flimmer
filaments (thicker, stiffer hairs are called mastigonemes).
These
filaments change flagellar action so that a wave moving down the filament
toward the tip pulls the cell along instead of pushing it. Such a flagellum often
is called a tinsel flagellum, whereas the naked flagellum is referred to as a
whiplash flagellum (figure 4.22). Cilia, on the other hand, normally have a
beat with two distinctive phases. In the effective stroke, the cilium strokes
through the surrounding fluid like an oar, thereby propelling the organism
along in the water.
The
cilium next bends along its length while it is pulled forward during the
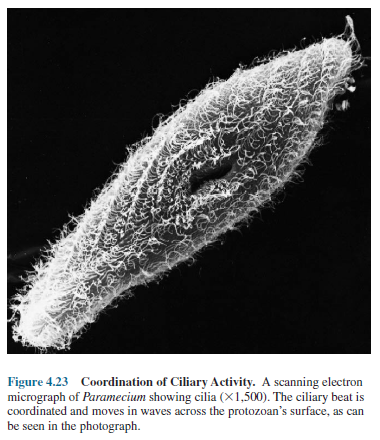
recovery stroke in preparation for another effective stroke. A ciliated
microorganism actually coordinates the beats so that some of its cilia are in
the recovery phase while others are carrying out their effective stroke (figure
4.23). This coordination allows the organism to move smoothly through the
water.
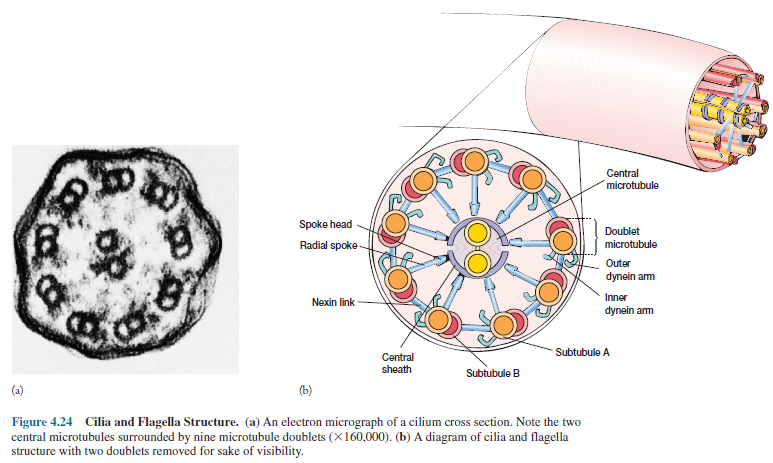
Despite their differences, cilia and flagella are very similar in ultrastructure. They are membrane-bound cylinders about 0.2 µm in diameter. Located in the matrix of the organelle is a complex, the axoneme, consisting of nine pairs of microtubule doublets arranged in a circle around two central tubules (figure 4.24). This is called the 9 + 2 pattern of microtubules.
Each doublet also has pairs
of arms projecting from subtubule A (the complete microtubule) toward a
neighboring doublet. A radial spoke extends from subtubule A toward the
internal pair of microtubules with their central sheath. These microtubules are
similar to those found in the cytoplasm. Each is constructed of two types of
tubulin subunits, α- and β-tubulins, that resemble the contractile protein
actin in their composition.
A basal
body lies in the cytoplasm at the base of each cilium or flagellum. It is a
short cylinder with nine microtubule triplets around its periphery (a 9 + 0
pattern) and is separated from the rest of the organelle by a basal plate. The
basal body directs the construction of these organelles. Cilia and flagella
appear to grow through the addition of preformed microtubule subunits at their
tips.
Cilia
and flagella bend because adjacent microtubule doublets slide along one another
while maintaining their individual lengths.
The
doublet arms (figure 4.24), about 15 nm long, are made of the protein dynein. ATP
powers the movement of cilia and flagella, and isolated dynein hydrolyzes ATP.
It appears that dynein arms interact with the B subtubules of adjacent doublets
to cause the sliding.
The
radial spokes also participate in this sliding motion. Cilia and flagella beat
at a rate of about 10 to 40 strokes or waves per second and propel
microorganisms rapidly. The record holder is the flagellate Monas
stigmatica, which swims at a rate of 260 µm/second (approximately 40 cell
lengths per second); the common euglenoid flagellate, Euglena gracilis, travels
at around 170 µm or 3 cell lengths per second. The ciliate protozoan Paramecium
caudatum swims at about 2,700 µm/second (12 lengths per second). Such
speeds are equivalent to or much faster than those seen in higher animals.
Comparison of
Procaryotic and Eucaryotic Cells
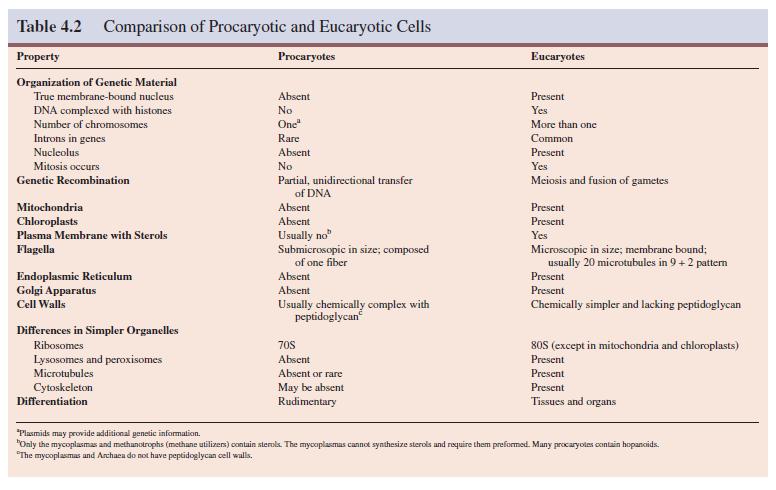
A
comparison of the cells in figure 4.25 demonstrates that there are many fundamental
differences between eucaryotic and procaryotic cells. Eucaryotic cells have a
membrane-enclosed nucleus. In contrast, procaryotic cells lack a true, membrane-delimited
nucleus. Bacteria and Archaea are procaryotes; all other organisms—algae, fungi,
protozoa, higher plants, and animals—are eucaryotic. Procaryotes normally are
smaller than eucaryotic cells, often about the size of eucaryotic mitochondria
and chloroplasts.
The presence of the eucaryotic nucleus is the most obvious difference between these two cell types, but several other major distinctions should be noted. It is clear from table 4.2 that procaryotic cells are much simpler structurally. In particular, an extensive and diverse collection of membrane-delimited organelles is missing.
Furthermore, procaryotes are simpler functionally in several
ways. They lack mitosis and meiosis, and have a simpler genetic organization.
Many complex eucaryotic processes are absent in procaryotes: phagocytosis and
pinocytosis, intracellular digestion, directed cytoplasmic streaming, ameboid
movement, and others.
Despite the many significant differences between these two basic cell forms, they are remarkably similar on the biochemical level as will be discussed in succeeding chapters. Procaryotes and eucaryotes are composed of similar chemical constituents. With a few exceptions the genetic code is the same in both, as is the way in which the genetic information in DNA is expressed.
The principles underlying metabolic processes and most of the more important metabolic pathways are identical. Thus beneath the profound structural and functional differences between procaryotes and eucaryotes, there is an even more fundamental unity: a molecular unity that is basic to all known life processes.











No comments:
Post a Comment