To obtain energy and construct new
cellular components, organisms must have a supply of raw materials or
nutrients.
Nutrients are substances used in biosynthesis and energy
production and therefore are required for microbial growth. This chapter
describes the nutritional requirements of microorganisms, how nutrients are
acquired, and the cultivation of microorganisms.
Environmental factors such as
temperature, oxygen levels, and the osmotic concentration of the medium are
critical in the successful cultivation of microorganisms. These topics are
discussed in chapter 6 after an introduction to microbial growth.
The Common
Nutrient Requirements of Microorganisms
Analysis of microbial cell composition
shows that over 95% of cell dry weight is made up of a few major
elements: carbon, oxygen, hydrogen, nitrogen, sulfur, phosphorus, potassium,
calcium, magnesium, and iron. These are called macroelements or
macronutrients because they are required by microorganisms in relatively large amounts.
The first six (C, O, H, N, S, and P) are components of carbohydrates, lipids,
proteins, and nucleic acids. The remaining four macroelements exist in the cell
as cations and play a variety of roles.
For example, potassium (K+)
is required for activity by a number of enzymes, including some of those
involved in protein synthesis. Calcium (Ca2+), among other
functions, contributes to the heat resistance of bacterial endospores.
Magnesium (Mg2+) serves as a cofactor for many enzymes, complexes
with ATP, and stabilizes ribosomes and cell membranes. Iron (Fe2+ and
Fe3+) is a part of cytochromes and a cofactor for enzymes and
electron-carrying proteins.
All organisms, including microorganisms,
require several micronutrients or trace elements besides
macroelements. The micronutrients—manganese, zinc, cobalt, molybdenum, nickel, and
copper—are needed by most cells. However, cells require such small amounts that
contaminants in water, glassware, and regular media components often are
adequate for growth. Therefore it is very difficult to demonstrate a
micronutrient requirement.
In nature, micronutrients are
ubiquitous and probably do not usually limit growth. Micronutrients are
normally a part of enzymes and cofactors, and they aid in the catalysis of
reactions and maintenance of protein structure. For example, zinc (Zn2+)
is present at the active site of some enzymes but is also involved in the
association of regulatory and catalytic subunits in E. coli aspartate carbamoyltransferase.
Manganese (Mn2+) aids many enzymes catalyzing the transfer of
phosphate groups. Molybdenum (Mo2+) is required for nitrogen
fixation, and cobalt (Co2+) is a component of vitamin B12.
Besides the common macro-elements and
trace elements, microorganisms may have particular requirements that reflect
the special nature of their morphology or environment. Diatoms (see figure
26.6c,d) need silicic acid (H4SiO4) to construct their beautiful
cell walls of silica [(SiO2)n]. Although most bacteria do not require
large amounts of sodium, many bacteria growing in saline lakes and oceans depend on the presence of high concentrations
of sodium ion (Na+).
Finally, it must be emphasized that
microorganisms require a balanced mixture of nutrients. If an essential
nutrient is in short supply, microbial growth will be limited regardless of the
concentrations of other nutrients.
Requirements for
Carbon, Hydrogen, and Oxygen
The requirements for carbon, hydrogen,
and oxygen often are satisfied together. Carbon is needed for the skeleton or
backbone of all organic molecules, and molecules serving as carbon sources normally
also contribute both oxygen and hydrogen atoms. They are the source of all
three elements. Because these organic nutrients are almost always reduced and
have electrons that they can donate to other molecules, they also can serve as
energy sources.
Indeed, the more reduced organic molecules
are, the higher their energy content (e.g., lipids have a higher energy content
than carbohydrates).
This is because, as we shall see
later, electron transfers release energy when the electrons move from reduced
donors with more negative reduction potentials to oxidized electron acceptors with
more positive potentials. Thus carbon sources frequently also serve as energy
sources, although they don’t have to.
One important carbon source that does
not supply hydrogen or energy is carbon dioxide (CO2). This is
because CO2 is oxidized and lacks hydrogen. Probably all
microorganisms can fix CO2—that is, reduce it and incorporate it
into organic molecules.
However, by definition, only autotrophs
can use CO2 as their sole or principal source of carbon. Many
microorganisms are autotrophic, and most of these carry out photosynthesis and
use light as their energy source. Some autotrophs oxidize inorganic molecules
and derive energy from electron transfers.
The reduction of CO2 is a
very energy-expensive process. Thus many microorganisms cannot use CO2
as their sole carbon source but must rely on the presence of more reduced,
complex molecules such as glucose for a supply of carbon. Organisms that use
reduced, preformed organic molecules as carbon sources are heterotrophs (these
preformed molecules normally come from other organisms). As mentioned
previously, most heterotrophs use reduced organic compounds as sources of both
carbon and energy.
For example, the glycolytic pathway
produces carbon skeletons for use in biosynthesis and also releases energy as
ATP and NADH. A most remarkable nutritional characteristic of microorganisms is
their extraordinary flexibility with respect to carbon sources. Laboratory
experiments indicate that there is no naturally occurring organic molecule that
cannot be used by some microorganism.
Actinomycetes will degrade amyl alcohol,
paraffin, and even rubber. Some bacteria seem able to employ almost anything as
a carbon source; for example, Burkholderia cepacia can use over 100
different carbon compounds. In contrast to these bacterial omnivores, some
bacteria are exceedingly fastidious and catabolize only a few carbon compounds.
Cultures of methylotrophic bacteria metabolize methane, methanol, carbon monoxide,
formic acid, and related one-carbon molecules. Parasitic members of the genus Leptospira
use only long-chain fatty acids as their major source of carbon and energy.
It appears that in natural
environments complex populations of microorganisms often will metabolize even
relatively indigestible human-made substances such as pesticides. Indigestible
molecules sometimes are oxidized and degraded in the presence of a growth-promoting
nutrient that is metabolized at the same time, a process called co-metabolism.
The products of this breakdown process can then be used as nutrients by other
microorganisms.
Nutritional Types
of Microorganisms
In addition to the need for carbon,
hydrogen, and oxygen, all organisms require sources of energy and electrons for
growth to take place.
Microorganisms can be grouped into
nutritional classes based on how they satisfy all these requirements (table
5.1). We have already seen that microorganisms can be classified as either
heterotrophs or autotrophs with respect to their preferred source of carbon.
There are only two sources of energy available to organisms: (1) light energy, and
(2) the energy derived from oxidizing organic or inorganic molecules.
Phototrophs use light as their energy source; chemotrophs
obtain energy from the oxidation of chemical compounds (either organic or
inorganic). Microorganisms also have only two sources for electrons. Lithotrophs
(i.e., “rock-eaters”) use reduced inorganic substances as their electron
source, whereas organotrophs extract electrons from organic compounds.
Despite the great metabolic diversity
seen in microorganisms, most may be placed in one of four nutritional classes
based on their primary sources of carbon, energy, and electrons (table 5.2).
The large majority of microorganisms
thus far studied are either photolithotrophic autotrophs or chemoorganotrophic
heterotrophs.
Photolithotrophic
autotrophs (often called photoautotrophs
or photolithoautotrophs) use light energy and have CO2 as their
carbon source. Eucaryotic algae and cyanobacteria employ water as the electron
donor and release oxygen. Purple and green sulfur bacteria cannot oxidize water
but extract electrons from inorganic donors like hydrogen, hydrogen sulfide,
and elemental sulfur.
Chemoorganotrophic
heterotrophs (often called chemoheterotrophs,
chemoorganoheterotrophs, or even heterotrophs) use organic compounds
as sources of energy, hydrogen, electrons, and carbon. Frequently the same
organic nutrient will satisfy all these requirements. It should be noted that
essentially all pathogenic microorganisms are chemoheterotrophs.
The other two nutritional classes have
fewer microorganisms but often are very important ecologically. Some purple and
green bacteria are photosynthetic and use organic matter as their electron donor
and carbon source. These photoorganotrophic heterotrophs (photoorganoheterotrophs)
are common inhabitants of polluted lakes and streams. Some of these bacteria
also can grow as photoautotrophs with molecular hydrogen as an electron donor.
The fourth group, the chemolithotrophic autotrophs (chemolithoautotrophs),
oxidizes reduced inorganic compounds such as iron, nitrogen, or sulfur
molecules to derive both energy and electrons for biosynthesis. Carbon dioxide
is the carbon source. A few chemolithotrophs can derive their carbon from
organic sources and thus are heterotrophic.
Chemolithotrophs contribute greatly to
the chemical transformations of elements (e.g., the conversion of ammonia to
nitrate or sulfur to sulfate) that continually occur in the ecosystem.
Although a particular species usually
belongs in only one of the four nutritional classes, some show great metabolic
flexibility and alter their metabolic patterns in response to environmental changes.
For example, many purple nonsulfur bacteria act as photoorganotrophic
heterotrophs in the absence of oxygen but oxidize organic molecules and
function chemotrophically at normal oxygen levels. When oxygen is low, photosynthesis
and oxidative metabolism may function simultaneously.
Another example is provided by
bacteria such as Beggiatoa that rely on inorganic energy sources and
organic or sometimes CO2) carbon sources. These microbes are sometimes
called mixotrophic because they combine chemolithoautotrophic and
heterotrophic metabolic processes.
This sort of flexibility seems complex
and confusing, yet it gives its possessor a definite advantage if environmental
conditions frequently change.
Requirements for Nitrogen, Phosphorus, and
Sulfur
To grow, a microorganism must be able to
incorporate large quantities of nitrogen, phosphorus, and sulfur. Although
these elements may be acquired from the same nutrients that supply carbon,
microorganisms usually employ inorganic sources as well.
Nitrogen is needed for the synthesis
of amino acids, purines, pyrimidines, some carbohydrates and lipids, enzyme
cofactors, and other substances. Many microorganisms can use the nitrogen in
amino acids, and ammonia often is directly incorporated through the action of
such enzymes as glutamate dehydrogenase or glutamine synthetase and glutamate
synthase. Most phototrophs and many non-photosynthetic microorganisms reduce
nitrate to ammonia and incorporate the ammonia in assimilatory nitrate
reduction. A variety of bacteria (e.g., many cyanobacteria and the symbiotic
bacterium Rhizobium) can reduce and assimilate atmospheric nitrogen
using the nitrogenase system.
Phosphorus is present in nucleic acids,
phospholipids, nucleotides like ATP, several cofactors, some proteins, and
other cell components. Almost all microorganisms use inorganic phosphate as
their phosphorus source and incorporate it directly.
Low phosphate levels actually limit
microbial growth in many aquatic environments. Phosphate uptake by E. coli has
been intensively studied. This bacterium can use both organic and inorganic
phosphate. Some organophosphates such as hexose 6-phosphates can be taken up
directly by transport proteins.
Other organophosphates are often
hydrolyzed in the periplasm by the enzyme alkaline phosphatase to produce
inorganic phosphate, which then is transported across the plasma membrane.
When inorganic phosphate is outside
the bacterium, it crosses the outer membrane by the use of a porin protein
channel. One of two transport systems subsequently moves the phosphate across
the plasma membrane. At high phosphate concentrations, transport probably is
due to the Pit system. When phosphate concentrations are low, the PST, (phosphate-specific
transport) system is more important. The PST system has higher affinity for
phosphate; it is an ABC transporter and uses a periplasmic binding protein.
Sulfur is needed for the synthesis of
substances like the amino acids cysteine and methionine, some carbohydrates,
biotin, and thiamine. Most microorganisms use sulfate as a source of sulfur and
reduce it by assimilatory sulfate reduction; a few require a reduced form of
sulfur such as cysteine.
Growth Factors of Microorganism
Microorganisms often grow and
reproduce when minerals and sources of energy, carbon, nitrogen, phosphorus,
and sulfur are supplied. These organisms have the enzymes and pathways necessary
to synthesize all cell components required for their wellbeing.
Many microorganisms, on the other
hand, lack one or more essential enzymes. Therefore they cannot manufacture all
indispensable constituents but must obtain them or their precursors from the
environment. Organic compounds required because they are essential cell
components or precursors of such components and cannot be synthesized by the
organism are called growth factors.
There are three major classes of
growth factors: (1) amino acids, (2)
purines and pyrimidines, and (3) vitamins. Amino acids are needed for
protein synthesis, purines and pyrimidines for nucleic acid synthesis. Vitamins
are small organic molecules that usually make up all or part of enzyme
cofactors, and only very small amounts sustain growth.
The functions of selected vitamins,
and examples of microorganisms requiring them, are given in table 5.3. Some
microorganisms require many vitamins; for example, Enterococcus faecalis needs
eight different vitamins for growth. Other growth factors are also seen; heme (from
hemoglobin or cytochromes) is required by Haemophilus influenzae, and
some mycoplasmas need cholesterol.
Knowledge of the specific growth
factor requirements of many microorganisms makes possible quantitative
growth-response assays for a variety of substances. For example, species from
the bacterial genera Lactobacillus and Streptococcus can be used
in microbiological assays of most vitamins and amino acids.
The appropriate bacterium is grown in
a series of culture vessels, each containing medium with an excess amount of
all required components except the growth factor to be assayed. A different amount
of growth factor is added to each vessel. The standard curve is prepared by
plotting the growth factor quantity or concentration against the total extent
of bacterial growth.
Ideally the amount of growth resulting
is directly proportional to the quantity of growth factor present; if the
growth factor concentration doubles, the final extent of bacterial growth
doubles. The quantity of the growth factor in a test sample is determined by
comparing the extent of growth caused by the unknown sample with that resulting
from the standards. Microbiological assays are specific, sensitive, and simple.
They still are used in the assay of substances like vitamin B12 and biotin,
despite advances in chemical assay techniques.
The observation that many
microorganisms can synthesize large quantities of vitamins has led to their use
in industry. Several water-soluble and fat-soluble vitamins are produced partly
or completely using industrial fermentations. Good examples of such vitamins and
the microorganisms that synthesize them are riboflavin (Clostridium,
Candida, Ashbya, Eremothecium), coenzyme A (Brevibacterium), vitamin
B12 (Streptomyces, Propionibacterium)
Uptake of Nutrients by the Cell
The first step in nutrient use is
uptake of the required nutrients by the microbial cell. Uptake mechanisms must
be specific—that is, the necessary substances, and not others, must be
acquired. It does a cell no good to take in a substance that it cannot use.
Since microorganisms often live in nutrient-poor habitats, they must be able to
transport nutrients from dilute solutions into the cell against a concentration
gradient. Finally, nutrient molecules must pass through a selectively permeable
plasma membrane that will not permit the free passage of most substances. In
view of the enormous variety of nutrients and the complexity of the task, it is
not surprising that microorganisms make use of several different transport
mechanisms.
The most important of these are
facilitated diffusion, active transport, and group translocation. Eucaryotic
microorganisms do not appear to employ group translocation but take up
nutrients by the process of endocytosis.
Facilitated Diffusion in Cell
A few substances, such as glycerol,
can cross the plasma membrane by passive diffusion. Passive diffusion, often
simply called diffusion, is the process in which molecules move from a region of
higher concentration to one of lower concentration because of random thermal agitation.
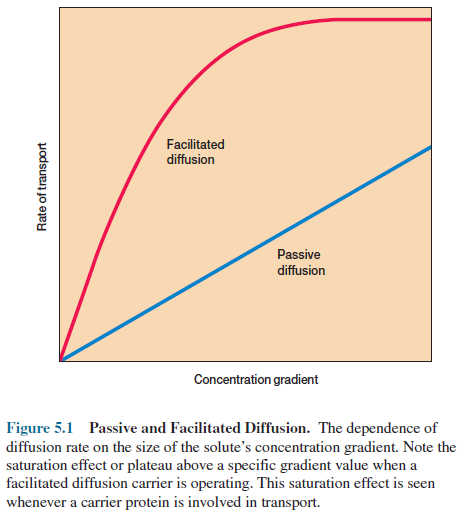
The rate of passive diffusion is dependent on the size of the concentration
gradient between a cell’s exterior and its interior (figure 5.1). A fairly
large concentration gradient is required for adequate nutrient uptake by
passive diffusion (i.e., the external nutrient concentration must be high), and
the rate of uptake decreases as more nutrient is acquired unless it is used
immediately. Very small molecules such as H2O, O2, and CO2
often move across membranes by passive diffusion. Larger molecules, ions, and
polar substances do not cross membranes by passive or simple diffusion.
The rate of diffusion across
selectively permeable membranes is greatly increased by using carrier proteins,
sometimes called permeases, which are embedded in the plasma membrane.
Because a carrier aids the diffusion
process, it is called facilitated diffusion. The rate of facilitated diffusion
increases with the concentration gradient much more rapidly and at lower
concentrations of the diffusing molecule than that of passive diffusion (figure
5.1). Note that the diffusion rate levels off or reaches a plateau above a
specific gradient value because the carrier is saturated— that is, the carrier
protein is binding and transporting as many solute molecules as possible. The
resulting curve resembles an enzyme-substrate curve and is different from the linear
response seen with passive diffusion.
Carrier proteins also resemble enzymes
in their specificity for the substance to be transported; each carrier is
selective and will transport only closely related solutes. Although a carrier
protein is involved, facilitated diffusion is truly diffusion. A concentration
gradient spanning the membrane drives the movement of molecules, and no
metabolic energy input is required. If the concentration gradient disappears,
net inward movement ceases.
The gradient can be maintained by
transforming the transported nutrient to another compound or by moving it to
another membranous compartment in eucaryotes. Interestingly, some of these
carriers are related to the major intrinsic protein of mammalian eye lenses and
thus belong to the MIP family of proteins. The two most widespread MIP channels
in bacteria are aquaporins that transport water and glycerol facilitators,
which aid glycerol diffusion.
Although much work has been done on
the mechanism of facilitated diffusion, the process is not yet understood
completely.
It appears that the carrier protein
complex spans the membrane (figure 5.2). After the solute molecule binds to the
outside, the carrier may change conformation and release the molecule on the cell
interior. The carrier would subsequently change back to its original shape and
be ready to pick up another molecule. The net effect is that a lipid-insoluble
molecule can enter the cell in response to its concentration gradient. Remember
that the mechanism is driven by concentration gradients and therefore is
reversible.
If the solute’s concentration is
greater inside the cell, it will move outward. Because the cell metabolizes
nutrients upon entry, influx is favored.
Facilitated diffusion does not seem to
be important in prokaryotes because nutrient concentrations often are lower
outside the cell so that facilitated diffusion cannot be used in uptake.
Glycerol is transported by facilitated
diffusion in E. coli, Salmonella typhimurium, Pseudomonas, Bacillus, and
many other bacteria.
The process is much more prominent in
eucaryotic cells where it is used to transport a variety of sugars and amino
acids.
Active Transport in Cell
Although facilitated diffusion carriers can efficiently move molecules to the interior when the solute concentration is higher on the outside of the cell, they cannot take up solutes that are already more concentrated within the cell (i.e., against a concentration gradient). Microorganisms often live in habitats characterized by very dilute nutrient sources, and, to flourish, they must be able to transport and concentrate these nutrients. Thus facilitated diffusion mechanisms are not always adequate, and other approaches must be used. The two most important transport processes in such situations are active transport and group translocation, both energy-dependent processes.
Active transport is the transport of
solute molecules to higher concentrations, or against a concentration gradient,
with the use of metabolic energy input. Because active transport involves protein
carrier activity, it resembles facilitated diffusion in some ways.
The carrier proteins or permeases bind
particular solutes with great specificity for the molecules transported.
Similar solute molecules can compete for the same carrier protein in both
facilitated diffusion and active transport. Active transport is also
characterized by the carrier saturation effect at high solute concentrations
(figure 5.1).
Nevertheless, active transport differs
from facilitated diffusion in its use of metabolic energy and in its ability to
concentrate substances. Metabolic inhibitors that block energy production will
inhibit active transport but will not affect facilitated diffusion (at least
for a short time).
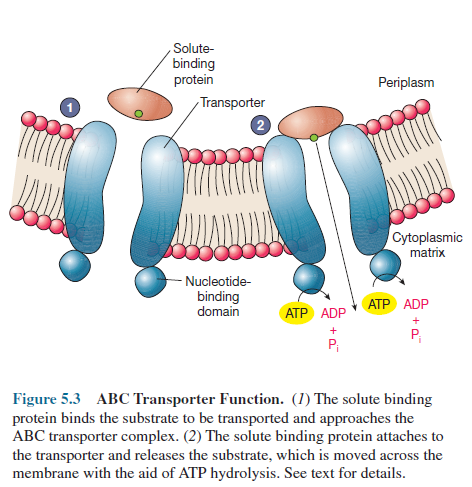
Binding protein transport systems or ATP-binding
cassette transporters (ABC transporters) are active in bacteria,
archaea, and eucaryotes. Usually these transporters consist of two hydrophobic membrane-spanning
domains associated on their cytoplasmic surfaces with two nucleotide-binding
domains (figure 5.3). The membrane-spanning domains form a pore in the membrane
and the nucleotide-binding domains bind and hydrolyze ATP to drive uptake. ABC
transporters employ special substrate binding proteins, which are located in
the periplasmic space of gram-negative bacteria (see figure 3.23) or are
attached to membrane lipids on the external face of the gram-positive plasma membrane.
These binding proteins, which also may
participate in chemotaxis, bind the molecule to be transported and then
interact with the membrane transport proteins to move the solute molecule
inside the cell. E. coli transports a variety of sugars (arabinose,
maltose, galactose, ribose) and amino acids (glutamate, histidine, leucine) by
this mechanism.
Substances entering gram-negative
bacteria must pass through the outer membrane before ABC transporters and other
active transport systems can take action. There are several ways in which this
is accomplished. When the substance is small, a generalized porin protein such
as OmpF can be used; larger molecules require specialized porins.
In some cases (e.g., for uptake of
iron and vitamin B12), specialized high-affinity outer membrane receptors and
transporters are used. It should be noted that eucaryotic ABC transporters are sometimes
of great medical importance. Some tumor cells pump drugs out using these
transporters. Cystic fibrosis results from a mutation that inactivates an ABC
transporter that acts as a chloride ion channel in the lungs.
Bacteria also use proton gradients
generated during electron transport to drive active transport. The membrane
transport proteins responsible for this process lack special periplasmic
solute-binding proteins. The lactose permease of E. coli is a
well-studied example. The permease is a single protein having a molecular weight
of about 30,000. It transports a lactose molecule inward as a proton
simultaneously enters the cell (a higher concentration of protons is maintained
outside the membrane by electron transport chain activity). Such linked
transport of two substances in the same direction is called symport.
Here, energy stored as a proton gradient
drives solute transport. Although the mechanism of transport is not completely
understood, it is thought that binding of a proton to the transport protein
changes its shape and affinity for the solute to be transported. E. coli also
uses proton symport to take up amino acids and organic acids like succinate and
malate.
A proton gradient also can power
active transport indirectly, often through the formation of a sodium ion
gradient. For example, an E. coli sodium transport system pumps sodium
outward in response to the inward movement of protons (figure 5.4). Such linked
transport in which the transported substances move in opposite directions is
termed antiport. The sodium gradient generated by this proton antiport system
then drives the uptake of sugars and amino acids.
A sodium ion could attach to a carrier
protein, causing it to change shape. The carrier would then bind the sugar or
amino acid tightly and orient its binding sites toward the cell interior.
Because of the low intracellular sodium concentration, the sodium ion would
dissociate from the carrier, and the other molecule would follow. E. coli transport
proteins carry the sugar melibiose and the amino acid glutamate when sodium simultaneously
moves inward.
Sodium symport or cotransport also is
an important process in eucaryotic cells where it is used in sugar and amino
acid uptake.
ATP, rather than proton motive force,
usually drives sodium transport in eucaryotic cells.
Often a microorganism has more than
one transport system for each nutrient, as can be seen with E. coli. This
bacterium has at least five transport systems for the sugar galactose, three
systems each for the amino acids glutamate and leucine, and two potassium
transport complexes. When there are several transport systems for the same
substance, the systems differ in such properties as their energy source, their
affinity for the solute transported, and the nature of their regulation.
Presumably this diversity gives its possessor an added competitive advantage in
a variable environment.
Group Translocation
In active transport, solute molecules
move across a membrane without modification. Many procaryotes also take up
molecules by group translocation, a process in which a molecule is transported into
the cell while being chemically altered (this can be classified as a type of
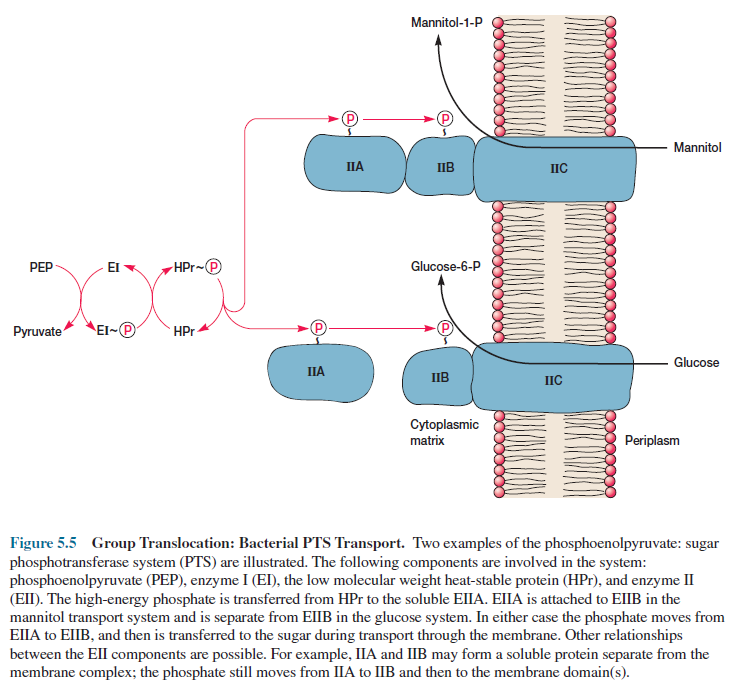
energy-dependent transport because metabolic energy is used). The best-known
group translocation system is the phosphoenolpyruvate: sugar phosphotransferase
system (PTS). It transports a variety of sugars into procaryotic cells while
phosphorylating them using phosphoenolpyruvate (PEP) as the phosphate donor.
PEP + sugar (outside) → pyruvate + sugar
-- P (inside)
The PTS is quite complex. In E.
coli and Salmonella typhimurium, it consists of two enzymes and a
low molecular weight heat-stable protein (HPr). HPr and enzyme I (EI) are
cytoplasmic.
Enzyme II (EII) is more variable in structure
and often composed of three subunits or domains. EIIA (formerly called EIII) is
cytoplasmic and soluble. EIIB also is hydrophilic but frequently is attached to
EIIC, a hydrophobic protein that is embedded in the membrane.
A high-energy phosphate is transferred
from PEP to enzyme II with the aid of enzyme I and HPr (figure 5.5). Then, a
sugar molecule is phosphorylated as it is carried across the membrane by enzyme
II.
Enzyme II transports only specific
sugars and varies with PTS, whereas enzyme I and HPr are common to all PTSs.
PTSs are widely distributed in
procaryotes. Except for some species of Bacillus that have both
glycolysis and the phosphotransferase system, aerobic bacteria seem to lack
PTSs. Members of the genera Escherichia, Salmonella, Staphylococcus, and
other facultatively anaerobic bacteria have phosphotransferase systems; some
obligately anaerobic bacteria (e.g., Clostridium) also have PTSs. Many carbohydrates
are transported by these systems.
E. coli takes up glucose, fructose, mannitol, sucrose,
N-acetylglucosamine, cellobiose, and other carbohydrates by group translocation.
Besides their role in transport, PTS proteins can act as chemoreceptors for
chemotaxis.
Iron Uptake by Cell
Almost all microorganisms require iron
for use in cytochromes and many enzymes. Iron uptake is made difficult by the
extreme insolubility of ferric iron (Fe3+) and its derivatives,
which leaves little free iron available for transport. Many bacteria and fungi
have overcome this difficulty by secreting siderophores [Greek for iron
bearers].
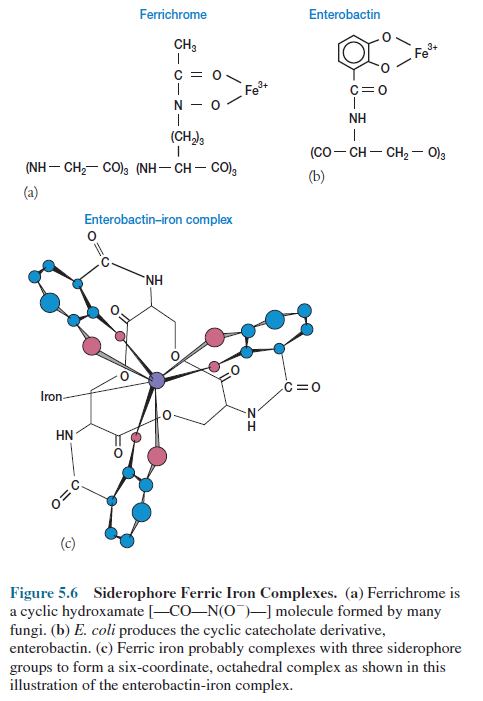
Siderophores are low molecular weight
molecules that are able to complex with ferric iron and supply it to the cell.
These iron-transport molecules are normally either hydroxamates or phenolatescatecholates.
Ferrichrome is a hydroxamate produced
by many fungi; enterobactin is the catecholate formed by E. coli (figure
5.6a,b). It appears that three siderophore groups complex with iron
orbitals to form a six-coordinate, octahedral complex (figure 5.6c).
Microorganisms secrete siderophores
when little iron is available in the medium. Once the iron-siderophore complex
has reached the cell surface, it binds to a siderophore-receptor protein.
Then the iron is either released to
enter the cell directly or the whole iron-siderophore complex is transported
inside by an ABC transporter. In E. coli the siderophore receptor is in
the outer membrane of the cell envelope; when the iron reaches the periplasmic
space, it moves through the plasma membrane with the aid of the transporter.
After the iron has entered the cell, it is reduced to the ferrous form (Fe2+).
Iron is so crucial to microorganisms that they may use more than one route of
iron uptake to ensure an adequate supply.
Culture Media in Microbiology
Much of the study of microbiology
depends on the ability to grow and maintain microorganisms in the laboratory,
and this is possible only if suitable culture media are available. A culture medium
is a solid or liquid preparation used to grow, transport, and store
microorganisms. To be effective, the medium must contain all the nutrients the
microorganism requires for growth.
Specialized media are essential in the
isolation and identification of microorganisms, the testing of antibiotic
sensitivities, water and food analysis, industrial microbiology, and other
activities.
Although all microorganisms need
sources of energy, carbon, nitrogen, phosphorus, sulfur, and various minerals,
the precise composition of a satisfactory medium will depend on the species one
is trying to cultivate because nutritional requirements vary so greatly.
Knowledge of a microorganism’s normal habitat often is useful in selecting an
appropriate culture medium because its nutrient requirements reflect its
natural surroundings.
Frequently a medium is used to select
and grow specific microorganisms or to help identify a particular species. In such
cases the function of the medium also will determine its composition.
Synthetic or Defined Culture Media
Some microorganisms, particularly photolithotrophic autotrophs such as cyanobacteria and eucaryotic algae, can be grown on relatively simple media containing CO2 as a carbon source (often added as sodium carbonate or bicarbonate), nitrate or ammonia as a nitrogen source, sulfate, phosphate, and a variety of minerals (table 5.4). Such a medium in which all components are known is a defined medium or synthetic medium.
Many chemoorganotrophic
heterotrophs also can be grown in defined media with glucose as a carbon source
and an ammonium salt as a nitrogen source. Not all defined media are as simple
as the examples in table 5.4 but may be constructed from dozens of components.
Defined media are used widely in research, as it is often desirable to know
what the experimental microorganism is metabolizing.
Complex Culture Media
Media that contain some ingredients of
unknown chemical composition are complex media. Such media are very useful, as
a single complex medium may be sufficiently rich and complete to meet the
nutritional requirements of many different microorganisms.
In addition, complex media often are
needed because the nutritional requirements of a particular microorganism are
unknown, and thus a defined medium cannot be constructed. This is the situation
with many fastidious bacteria, some of which may even require a medium
containing blood or serum.
Complex media contain undefined
components like peptones, meat extract, and yeast extract. Peptones are protein
hydrolysates prepared by partial proteolytic digestion of meat, casein, soya meal,
gelatin, and other protein sources. They serve as sources of carbon, energy,
and nitrogen. Beef extract and yeast extract are aqueous extracts of lean beef
and brewer’s yeast, respectively.
Beef extract contains amino acids,
peptides, nucleotides, organic acids, vitamins, and minerals. Yeast extract is
an excellent source of B vitamins as well as nitrogen and carbon compounds.
Three commonly used complex media are (1) nutrient broth, (2) tryptic soy
broth, and (3) MacConkey agar (table 5.5).
If a solid medium is needed for
surface cultivation of microorganisms, liquid media can be solidified with the
addition of 1.0 to 2.0% agar; most commonly 1.5% is used. Agar is a sulfated
polymer composed mainly of D-galactose, 3,6-anhydro-L-galactose, and D-glucuronic
acid (Box 5.1). It usually is extracted from red algae (see figure 26.8).
Agar is well suited as a solidifying agent because after it has been melted in
boiling water, it can be cooled to about 40 to 42°C before hardening and will
not melt again until the temperature rises to about 80 to 90°C. Agar is also an
excellent hardening agent because most microorganisms cannot degrade it.
Other solidifying agents are sometimes
employed. For example, silica gel is used to grow autotrophic bacteria on solid
media in the absence of organic substances and to determine carbon sources for heterotrophic
bacteria by supplementing the medium with various organic compounds.
Types of Culture Media
Culture media such as tryptic soy
broth and tryptic soy agar are called general purpose media because they
support the growth of many microorganisms. Blood and other special nutrients
may be added to general purpose media to encourage the growth of fastidious heterotrophs.
These specially fortified media (e.g., blood agar) are called enriched media.
Selective media favor the growth of
particular microorganisms. Bile salts or dyes like basic fuchsin and crystal
violet favor the growth of gram-negative bacteria by inhibiting the growth of gram-positive
bacteria without affecting gram-negative organisms.
Endo agar, eosin methylene blue agar,
and MacConkey agar (table 5.5), three media widely used for the detection of E.
coli and related bacteria in water supplies and elsewhere, contain dyes that
suppress gram-positive bacterial growth. MacConkey agar also contains bile
salts. Bacteria also may be selected by incubation with nutrients that they
specifically can use. A medium containing only cellulose as a carbon and energy
source is quite effective in the isolation of cellulose-digesting bacteria. The
possibilities for selection are endless, and there are dozens of special selective
media in use.
Differential media are media that
distinguish between different groups of bacteria and even permit tentative
identification of microorganisms based on their biological characteristics.
Blood agar is both a differential
medium and an enriched one. It distinguishes between hemolytic and nonhemolytic
bacteria. Hemolytic bacteria (e.g., many streptococci and staphylococci
isolated from throats) produce clear zones around their colonies because of red
blood cell destruction. MacConkey agar is both differential and selective.
Since it contains lactose and neutral red dye, lactose-fermenting colonies
appear pink to red in color and are easily distinguished from colonies of non-fermenters.
Isolation of Pure Cultures
In natural habitats microorganisms
usually grow in complex, mixed populations containing several species. This
presents a problem for the microbiologist because a single type of
microorganism cannot be studied adequately in a mixed culture. One needs a pure
culture, a population of cells arising from a single cell, to characterize an
individual species. Pure cultures are so important that the development of pure
culture techniques by the German bacteriologist Robert Koch transformed
microbiology.
Within about 20 years after the
development of pure culture techniques most pathogens responsible for the major
human bacterial diseases had been isolated (see Table 1.1). There are
several ways to prepare pure cultures; a few of the more common approaches are
reviewed here.
The Spread Plate and Streak Plate Culture Method
If a mixture of cells is spread out on
an agar surface so that every cell grows into a completely separate colony, a
macroscopically visible growth or cluster of microorganisms on a solid medium, each
colony represents a pure culture. The spread plate is an easy, direct way of
achieving this result. A small volume of dilute microbial mixture containing
around 30 to 300 cells is transferred to the center of an agar plate and spread
evenly over the surface with a sterile bent-glass rod (figure 5.7). The
dispersed cells develop into isolated colonies. Because the number of colonies should
equal the number of viable organisms in the sample, spread plates can be used
to count the microbial population.
Pure colonies also can be obtained
from streak plates. The microbial mixture is transferred to the edge of an agar
plate with an inoculating loop or swab and then streaked out over the surface in
one of several patterns (figure 5.8). At some point in the process, single
cells drop from the loop as it is rubbed along the agar surface and develop
into separate colonies (figure 5.9). In both spread-plate and streak-plate
techniques, successful isolation depends on spatial separation of single cells.
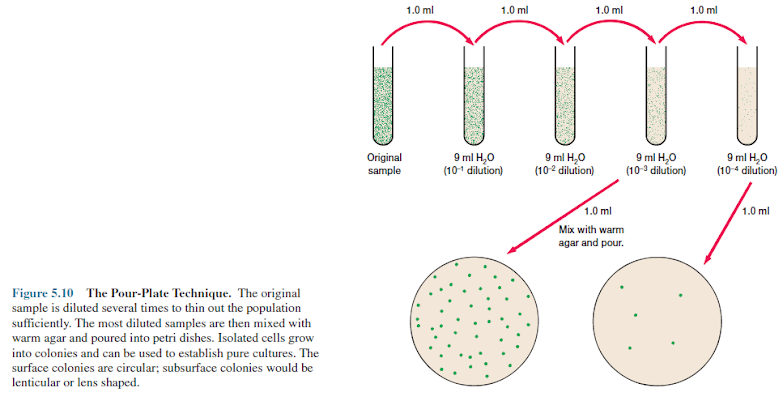
The Pour Plate Culture Method
Extensively used with bacteria and fungi, a pour plate also can yield isolated colonies. The original sample is diluted several times to reduce the microbial population sufficiently to obtain separate colonies when plating (figure 5.10). Then small volumes of several diluted samples are mixed with liquid agar that has been cooled to about 45°C, and the mixtures are poured immediately into sterile culture dishes.
Most
bacteria and fungi are not killed by a brief exposure to the warm agar. After
the agar has hardened, each cell is fixed in place and forms an individual colony.
Plates containing between 30 and 300 colonies are counted. The total number of
colonies equals the number of viable microorganisms in the diluted sample.
Colonies growing on the surface also can be used to inoculate fresh medium and
prepare pure cultures (Box 5.2).
The preceding techniques require the
use of special culture dishes named petri dishes or plates after their inventor
Julius Richard Petri, a member of Robert Koch’s laboratory; Petri developed these
dishes around 1887 and they immediately replaced agar-coated glass plates. They
consist of two round halves, the top half overlapping the bottom (figure 5.8).
Petri dishes are very easy to use, may be stacked on each other to save space,
and are one of the most common items in microbiology laboratories.
Colony Morphology and Growth
Colony development on agar surfaces aids the microbiologist in identifying bacteria because individual species often form colonies of characteristic size and appearance (figure 5.11).
When a mixed population has been
plated properly, it sometimes is possible to identify the desired colony based
on its overall appearance and use it to obtain a pure culture. The structure of
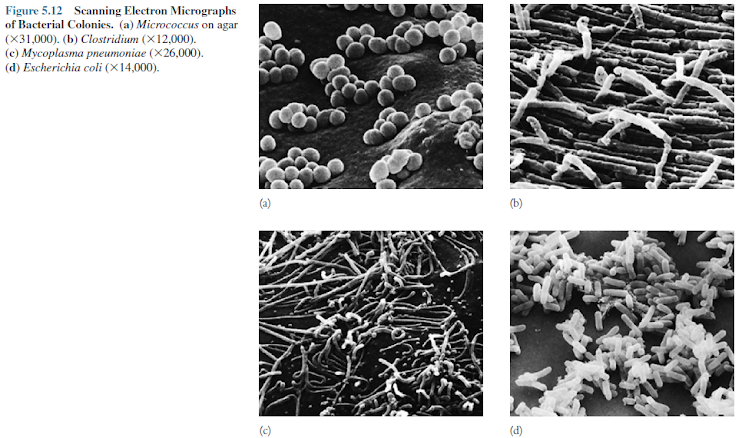
bacterial colonies also has been examined with the scanning electron microscope.
The microscopic structure of colonies is often as variable as their visible
appearance (figure 5.12).
In nature bacteria and many other
microorganisms often grow on surfaces in biofilms. However, sometimes they do
form discrete colonies. Therefore an understanding of colony growth is important,
and the growth of colonies on agar has been frequently studied.
Generally the most rapid cell growth
occurs at the colony edge. Growth is much slower in the center, and cell
autolysis takes place in the older central portions of some colonies. These
differences in growth appear due to gradients of oxygen, nutrients, and toxic
products within the colony. At the colony edge, oxygen and nutrients are
plentiful. The colony center, of course, is much thicker than the edge.
Consequently oxygen and nutrients do not diffuse readily into the center, toxic
metabolic products cannot be quickly eliminated, and growth in the colony
center is slowed or stopped. Because of these environmental variations within a
colony, cells on the periphery can be growing at maximum rates while cells in
the center are dying.
It is obvious from the colonies pictured in figure 5.11 that bacteria growing on solid surfaces such as agar can form quite complex and intricate colony shapes. These patterns vary with nutrient availability and the hardness of the agar surface. It is not yet clear how characteristic colony patterns develop. Nutrient diffusion and availability, bacterial chemotaxis, and the presence of liquid on the surface all appear to play a role in pattern formation. Undoubtedly cell cell communication and quorum sensing is important as well. Much work will be required to understand the formation of bacterial colonies and biofilms.












No comments:
Post a Comment